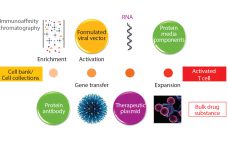
The concept of gene therapy is far from new, with initial studies performed over 20 years ago (1). However, in the past few years an explosion of interest in this area has gone beyond initial regenerative approaches using viral vectors. Interest is now moving increasingly into potential use of T cells modified using recombinant viral vectors for immunotherapy applications. Such therapies are based on using either adenoassociated virus (AAV) or lentivirus (1), both vectors being frequently generated through transient expression…
2016
Innovative Downstream Purification Solutions for Viral Vectors: Enabling Platform Approaches to Advance Gene Therapies
Over the past decade, gene therapy applications and their importance in the biopharmaceutical industry have been increasing. Gene therapies promise versatile treatment options that could revolutionize and transform medicine. As treatment modalities, they offer the possibility of long-term and potentially curative benefits to patients with genetic or acquired diseases. Gene therapies are designed to treat disease by delivering genetic material that encodes a protein with a therapeutic effect into a patient’s cells. It can be used to replace a missing…
Designing the Optimal Manufacturing Strategy for an Adherent Allogeneic Cell Therapy
Cell therapies (CTs) offer potential treatments for a wide range of medical conditions (1–6) by replacing cells, repairing tissues affected by either disease or damage (7), or delivering genetic or molecular agents that promote self-healing (8). CT research and development is continuously growing (9), with increasing numbers of CT candidates reaching phase 3 clinical trials (9–11). Developers aim to make products that can survive in a competitive landscape while complying with stringent regulatory requirements to control the quality and safety…
October 2016 From the Editor
Every September, we editors step onto a virtual slide that takes us into the new year with increasing steepness and velocity. Here come the fall supplements and special projects! And as the new year approaches, our momentum is often halted abruptly by the November and December holiday schedules, during which many of our contacts seem to vanish. The highlight of our fall season is always the BioProcess International Conference and Exposition in Boston, this year occurring the very week that…
October Spotlight
Adaptive Pathways Pilot Program Results A final report became available this past summer about the European Medicines Agency’s (EMA) pilot project on developing medicines for unmet needs. The project showed that adaptive pathways can bring multiple stakeholders together — including regulators, health-technology assessment (HTA) bodies, healthcare professionals, and patients — to evaluate drug development and data gathering. Adaptive pathways allow for a planned, progressive approach to bringing medicines to patients. The concept makes use of existing approval tools (e.g., conditional…
Biosimilar Therapeutic Monoclonal Antibodies: Gaps in Science Limit Development of an Industry Standard for Their Regulatory Approval, Part 1
Biosimilars are biologically derived pharmaceuticals intended to have clinical similarity to a legally marketed innovator product when that product’s patent or market exclusivity has expired. By contrast with generic small-molecule drugs, clinical performance of a biologic pharmaceutical is a function of its structural complexity and higher-order structure (HOS). Biomanufacturing controls of such complex products cannot fully ensure chemical similarity between an innovator product and putative biosimilar because minor differences in chemical modifications and HOS can significantly alter a product’s safety…
Viral Risk Evaluation of Raw Materials Used in Biopharmaceutical Production
Ensuring a continuous supply of safe medicines to patients is a key objective for both health authorities and the pharmaceutical industry. A critical component to that end is maintaining a reliable supply of qualified raw materials (RMs). Manufacturers must ensure not only the suitability of RMs for their intended use in a manufacturing process, but also their highest attainable safety with regards to viruses and other adventitious agents. The need to apply a risk-based RM control strategy is in line…
Emerging Technology Trends in Biologics Development: A Contract Development and Manufacturing Perspective
For a contract development and manufacturing organization (CDMO), process development and manufacturing of recombinant proteins must be linked because of tight timelines driven by client expectations. Those are in turn driven by a need for rapid progression to clinical testing. Early in process development, the choice of raw materials needs to reflect existing supply chain and manufacturing infrastructure, but remain suitable for scaling up to meet future needs. One approach is to establish platform processes for a class of molecules…
Selective and Flexible Chromatography Media: Improving Biopharmaceutical Operational Efficiencies
Continuing development in protein and peptide engineering have produced a broad range of new biological products with improved therapeutic and diagnostic potential. In the development pipeline, more than 900 biologic products target more than 100 diseases (1). Increased manufacturing complexities caused by closely related impurities and requirements to improve process efficiencies and reduce operating costs highlight the need for new approaches in protein purification. Platform-based chromatographic approaches have been successfully applied in separating and purifying monoclonal antibody (MAb) products. But…
Viral Clearance in Antibody Purification Using Tentacle Ion Exchangers
Manufacturers strive toward cost-effective purification of target molecules and a high level of confidence that their biologics are safe and not compromised by the presence of endogenous retrovirus-like particles or adventitious viruses (1). Reliable reduction of viral particles throughout downstream purification processes must be ensured through different techniques such as chemical treatment, filtration, and chromatography. Common monoclonal antibody (MAb) purification schemes use both cation- and anion-exchange chromatography steps (CEX, AEX). Although CEX (to remove product- and process-related impurities) is not…