India’s position as a global participant in small-molecule generic drugs, vaccines, and enzymes has been proven over decades. The country is one of the most populous and fastest-growing regions in the world, both economically and technically. But India’s potential as a biologics participant has not been realized. Its competence as a global biologics producer has not yet caught up. Global industry concerns regarding the country’s position in the (bio) pharmaceutical industry haven’t changed much over the past eight years since…
2016
Funding for Life-Science Ventures: Accelerating Innovation in Tools and Services
As a cofounder of Wave Biotech (now a division of GE Healthcare), my partners and I often struggled with critical choices regarding partnering and funding opportunities. Every new, attractive, and potentially disruptive technology will court attention once it experiences some modest adoption and acceptance, even while attempting to “fly under the radar” of major players. The challenge for life-science entrepreneurs is how best to navigate those decisions and select the right path as company founders. Weighing and evaluating potential partners…
The 2016 BPI Awards: Honoring the People, Organizations, and Technologies in Global Biotherapeutics
Since 2003, the mission of BioProcess International has been to connect biopharmaceutical scientists and decision makers to the science, technology, and expertise that can positively influence and improve existing bioprocesses. Our BioProcess International Awards were created in 2012 to mark the magazine’s 10-year anniversary. They allow us to reflect on and help honor the time and investment companies put into researching, developing, and launching biopharmaceutical products, technologies, and services to deliver better, more efficient treatments and increased hope to a…
Postapproval Changes for Biopharmaceutical Drug-Substance and Drug-Product Manufacture: Regulatory Complexity and Impact
Pharmaceutical products save or improve the lives of millions of people each year. Thorough regulatory review of chemistry, manufacturing, and controls (CMC) information is critical to ensure drug product safety, quality, and efficacy as well as to secure patients’ continuous access to such products. But achieving all of that at an effective cost is difficult. Companies race to launch products to patients as soon as possible after clinical efficacy is demonstrated. Biomanufacturers often need to make changes such as increasing…
Reducing Clinical-Phase Manufacturing Costs: Collaborating for Savings without Compromising Quality or Performance
In downstream purification of monoclonal antibodies (MAbs), the single greatest contributor to manufacturing costs is the expensive capture step typically based on protein A affinity chromatography. Almost since its introduction to bioprocessing, efforts have been made to reduce the cost of this step. Several alternative ligands have been promulgated as potential replacements for protein A, but they have proven difficult to adopt and scale up. Supplier companies have pushed for increases in capacity and economics, but those are always accompanied…
Medium and Feed Development: Beyond Maximizing Protein Titer to Optimizing Glycan Distribution and Simplifying Process Scale-Up
Serena Fries Smith, associate director of global technical engagements for Thermo Fisher Scientific, covered advantages in feed solutions in the context of current industry trends in an early November webinar. Leveraging feed designs and strategies can optimize glycosylation of complex proteins, simplify scale-up of fed-batch processes, and improve expression titers. Smith’s Presentation In 1994, the average expression titer was 0.5 g/L. Thanks in part to improvements in culture feeding, titers had increased to 1.3 g/L in 2004. By 2014, they…
Enabling Custom Solutions for Downstream Processing of Future Therapies: An Adenoassociated Virus Case Study
Orjana Terova is a purification product manager in the bioproduction division of Thermo Fisher Scientific. In a BPI webinar on 9 December 2016, she discussed the company’s custom resin program for purification of biological products. Thermo Fisher Scientific has dedicated a pilot-plant facility for this program. Terova’s Presentation Speed is the main development driver in downstream processing, but quality and efficiency are always critical. Purification processes need the highest resolution, capacity, salt tolerance, and operation speed possible. Consistency and reproducibility…
Improving Single-Use Bioreactor Design and Process Development: New Research Toward Intensifying Seed-Train and Scale-Up Methods Using 5:1 Turn-Down
In an early October webcast, Surendra Balekai (senior global product manager for bioproduction at Thermo Scientific) discussed improving bioreactor design for a 5:1 turn-down ratio. Balekai has worked for over 17 years with biological manufacturers in designing processing equipment for developing vaccines, blood products, and recombinant proteins. Balekai’s Presentation When bioreactors operate at a 5:1 ratio (20% volume), the primary challenges are carbon dioxide build-up in the headspace and fluid mixing at low volume. The CO2 produced will significantly affect…
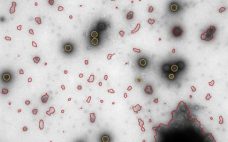
Reveal Information That Provides Insights: New Approaches to Subvisible Particle Characterization
In an October webcast, Vironova’s Josefina Nilsson (head of electron microscopy services) and Gustaf Kylberg (MiniTEM product manager) discussed subvisible particle characterization using the MiniTEM system, which provides both morphology and quantitative data. Nilsson and Kylberg’s Presentation Vironova combines expertise in electron microscopy and image analysis with in-house designed software. Electron microscopy is a good technique for getting to know your product in detail (morphology and other characteristics). After discussions with partners and clients, Vironova worked with Delong Instruments to…
The First Single-Use Diaphragm Valve: Automated and Controllable Systems Increase Process Reliability
Single-use components and systems now are firmly established in the pharmaceutical and biotechnology industries. The trend toward simplified and flexible upstream and downstream plant design means that these components are becoming increasingly important — especially in biopharmaceutical production. In the past, the only available disposables were primarily tubes, fittings, and possibly filters. But the number of single-use systems has been increasing for a number of years now. It is hardly surprising that plant designers and operators now can rely on…