Production of recombinant proteins usually happens in suspension cultures, with oxygen limitation playing a major role. Oxygen and nutrition feeds are of great significance to aerobic suspension cultures. Oxygen is often the controlling factor in orbital shaken systems because oxygen transfer occurs only through diffusion, which is limited by gas-exchange surface and mixing characteristics. Here, we compare growth characteristics of microbial cultures in a standard shaken incubator with those of cultures in a RAMbio fermentation system, paying particular attention to…
2016
A Turning Point for US Biosimilars
The next 12–18 months could be a critical time for biosimilars in the United States (1). This product class has grown rapidly since passage of the Biosimilars Price Competition and Innovation Act (BPCIA) of 2010. That landmark legislation allowed for biosimilar market approvals based on previously approved “reference products,” creating an expedited pathway that reduces biosimilar development costs and speeds regulatory review so patients get faster access to essential medicines. Increased competition from biosimilars could save the US healthcare system…
Factories of the Future: Can Patient-Specific Cell Therapies Get There from Here?
In many ways, patient-specific cell therapies (PSCTs) are still the “new kid on the block” in medicine. Researchers, therapeutic developers, manufacturers, regulators, and payers are still exploring and developing an understanding of the powerful benefits and unique challenges associated with this growing industry. As we all become more familiar with PSCTs, an evolution will need to occur — as it has for automobiles, computers, and every technological advance in human history — for these therapies to become widely adopted, cost-efficient,…
Emerging Platform Bioprocesses for Viral Vectors and Gene Therapies
Recent advances in molecular biology are expediting genomic sequencing to underpin precision medicine. Such progress is positioning gene and gene-modified cell therapy on the cusp of an extraordinary revolution in patient care for presently unmet medical needs — and a new therapeutic class that could rival monoclonal antibodies (MAbs) in importance. However, despite substantial strides made in clinical trials, the bioprocessing community is struggling to fulfill growing demands for biomanufacturing capacity to make gene and gene-modified cell therapies — including…
Cancer Immunotherapies: Fulfilling the Promise of Protein and Cell Therapies
With few exceptions, both small-molecule and biological cancer treatments have contributed only incrementally towards achieving long-term responses or outright cures. In this regard, emerging cell- and protein-based cancer immunotherapies represent game-changing strategies for treating even refractory cancer. With long-term responses now possible, medical science may be on the verge of delivering on the long-unfulfilled promise of making cancer a manageable disease. But impediments to commercializing cancer immunotherapies are substantial. Producing cell-based treatments entails substantial hands-on manipulation and perfecting the logistics…
Automation in Cell Therapy Manufacturing
The concept of automation conjures up images of robots on assembly lines or perhaps automobiles replacing horse-drawn carriages. In both examples, automation provides an ability to work tirelessly, with reproducible high-quality outputs at increased speed. For cell therapy, automation can be used to increase the scale of cell culture operations (e.g., bioreactors replacing flasks) and allow the use of closed systems that can protect cell products from contamination with adventitious agents from the environment or operators themselves. Closed systems also…
Automation of CAR-T Cell Adoptive Immunotherapy Bioprocessing: Technology Opportunities to Debottleneck Manufacturing
Continued clinical efficacy demonstrations of cell-based immunotherapies (iTx) such as chimeric antigen receptor T cell (CAR-T) therapies has made the prospect increasingly likely of an immunotherapy product achieving conditional market authorization in the short term. For example, Novartis and the University of Pennsylvania’s lead candidate (CTL019) for treating a range of hematological malignancies received breakthrough status from the US Food and Drug Administration (FDA) in 2014, permitting access to an expedited drug development pathway for high unmet medical needs (1).…
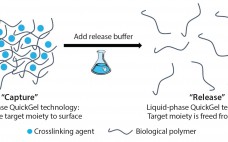
Development of a Novel Cell-Separation Platform: Discussion with Quad Technologies CEO Sean Kevlahan
Releasing and separating cells from surfaces and capture molecules are critical steps in cell therapy development. Research into such therapies as chimeric antigen receptor T cells (CAR-T) cancer therapies and stem-cell regenerative medicines demand the isolation and purification of viable and functional target cells. A number of cell-separation strategies can be used to produce such cells, but they are not able to deliver the required efficiency or scalability and can also cause damage to cells or affect their phenotype. Since…
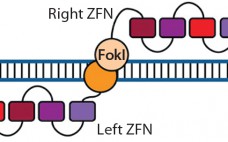
Therapeutic Gene Editing: Tools to Facilitate Basic Science or Stimuli for a Paradigm Shift in Biomanufacturing?
Historically, fundamental science and process engineering were separated by distinct vernaculars and a decade or more in the translation pathway of candidate therapeutics from laboratory to bedside (1). This crude metric holds true for the origins of the modern pharmaceutical industry, namely fine chemicals that supported the high-margin small molecules that constitute the majority of the pharmacopoeia even today. But as illustrated by deeply interwoven careers, companies, and technologies — including those related to monoclonal antibodies (MAbs) — that classic…
Collaboration Will Drive Regenerative Medicine: Toronto Development Center Will Help to Advance the Field
With support from the federal government of Canada, GE Healthcare and the Centre for Commercialization of Regenerative Medicine (CCRM) are pushing into new frontiers to advance the progress of cell therapy and regenerative medicine. When I first met Michael May, president and chief executive officer (CEO) of CCRM, both our organizations had been exploring opportunities in parallel to drive the cell therapy industry forward. CCRM’s mission is to create and sustain a global nexus for company creation, technology and cell…