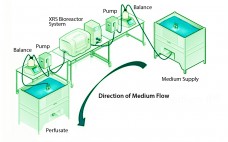
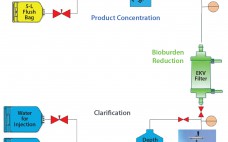
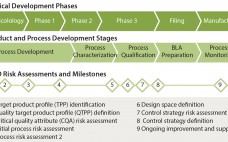
Although implementation of continuous manufacturing for biopharmaceuticals is in the early stages, continuous cell culture technology has been around for close to thirty years. Perfusion was initially developed in the late 1980s as a means for increasing protein titers (1). However, high costs driven by media consumption limited widespread commercial adoption. In the same time frame, advances in cell line engineering, media composition, and bioreactor design led to 10-fold increases in titers for batch and fed-batch modes, eliminating the first…
2016
Flexible Automation for Continuous Unit Operations
Continuous processing has the potential to provide significant cost and time savings for biopharmaceutical manufacturing, but that potential can be realized only if appropriate automation solutions are available for continuous flow between disparate upstream and downstream operations. Pall Life Sciences’ Allegro MVP system, a fully automated bioprocessing system designed for use in upstream and downstream single-use processing, enables flexible automation and thus facilitates continuous biopharmaceutical manufacturing. This article presents the results obtained using the Allegro MVP system in combination with…
From the Editor
Audience: the key element behind all style and content decisions in any spoken or written material. When publications existed only in print, audience-related criteria were easier to maintain than they are these days. Bound periodicals lived in libraries sequestered by subject. Unless you knew where “your” material lived, you were unlikely to stumble upon it — but information could get lost. Manual crossreferencing wasn’t easy, and I would not want to return to the days before powerful Internet search engines!…
Spotlight
Infectious Agents: A Global Effort Worldwide Commitment to Fight Antimicrobial Resistance: Medical, business, and government officials from around the world gathered in Amsterdam for the ECCMID 2016 Conference in April 2016. Hosted by the European Society of Clinical Microbiology and Infectious Diseases (ESCMID), the meeting reinforced its attendees’ commitment to fight antimicrobial resistance in a concerted effort. During an interactive session hosted by ESCMID and the Netherlands Society of Medical Microbiology (NVMM), researchers and representatives of the Dutch healthcare administration…
Bacteriophages, an Alternative to Antibiotics: Challenges and Possible Solutions for Bringing Them to Market
Bacteriophages are viruses (consisting of a genome contained within a protein coat) that specifically infect bacteria. They are the most abundant living entities on earth — the estimates range from 1030 to 1032 in total — and play key roles in regulating the microbial balance in every ecosystem where that has been explored (1). Bacteriophages are genotypic and phenotypically different from viruses that infect Archaea (Archaeovirus) and Eukarya (Eukaryovirus). The name bacteriovirus has been proposed as scientifically more accurate (2).…
Bridging Polymer Science and Biotechnology Applications with Single-Use Technologies
Implementation of single-use technology in the biotechnology industry is increasing every year. One major interest has been understanding the interaction of extractables with protein and cells for applications ranging from cell banking to biopharmaceutical manufacturing. In October 2015, the Engineering Conference International (ECI) organization hosted a conference in Leesburg, VA, to explore how the science of plastic applies to bioprocessing. The “Single-Use Technologies: Bridging Polymer Science to Biotechnology Applications” meeting brought together experts from different fields to share issues, understanding,…
Quality By Design for Monoclonal Antibodies, Part 1: Establishing the Foundations for Process Development
The quality by design (QbD) modernized approach to pharmaceutical development is intended to provide regulatory flexibility, increased development and manufacturing efficiency, and greater room to innovate as well as improve manufacturing processes within defined ranges without obtaining regulatory approval first. QbD is a systematic developmental approach that starts with a clear goal in mind and emphasizes understanding of how variability in both process and materials affects a final product (1). Historically, product quality has been assured either with end-product testing…
Osmolality Measurements for High-Concentration Protein–Polymer Solutions: Variation Based on Working Principles of Osmometers
Osmolality is a critical attribute for injectable formulations. It is desirable to have products match physiological osmotic conditions. Furthermore, osmolality provides confirmation of soluble content in solution. Preventing injection of hypo- or hyperosmotic solutions is a key element of parenteral formulation development. Additionally, some investigators have explored correlations between injection pain and formulation osmolality, although no significant correlation has yet been observed (1–4). Osmolality is a valuable in-process test also because it provides a reliable and repeatable value that reflects…
Enhanced Biosimilar Product Characterization: A Case Study Using Raman Spectroscopy Combined with Dynamic Light Scattering
Biophysical characterization has drawn great attention from the biopharmaceutical industry and regulatory agencies across the globe, especially for use in biosimilar drug product development. Currently available biophysical characterization tools can help in screening and optimizing better (more stable) formulations for such products. However, most tools cannot be used for head-to-head comparison of the biophysical properties of an optimized biosimilar formulation with those of an innovator product at higher concentrations. We developed and optimized a formulation for monoclonal antibody MAb B…
A Single-Use, Clinical-Scale Filling System: From Design to Delivery
Single-use components have been successfully incorporated into many unit operations for both upstream and downstream processing, from laboratory scale to commercial manufacturing. The development of single-use filling needles has created an opportunity to introduce fully disposable systems into final formulation and filling of drug products (1). One major challenge in replacing a cleanable filling line containing stainless steel needles is to ensure that an alternative system can satisfy all critical performance parameters established for an existing process. In 2012, Merck…