Standardizing equipment in biopharmaceutical production streamlines operations, saving end users time and money. An area ripe for standardization is the use of genderless connections, those in which the connector halves are identical in design. Historically, single-use connections have involved male and female halves, but using two different parts can create several inefficiencies and complications, such as Increased ordering complexity Greater risk of specifying the wrong system or connection, resulting in downtime and last-minute adjustments Longer lead times Increased stocking requirements…
2016
Increased Clarification Capacity: Using Filter Aid and FILTRODISCâ„¢ BIO SD for an Economical Filtration
Clarification of fermentation broths is one of the most important steps in bioprocessing. The first purification step after fermentation is the cell harvest, which is designed to remove cells and cell debris as well as to reach maximum product yield in compliance with existing regulatory environments. Standard technologies (centrifugation, separators, membrane, and depth filtration) can no longer handle the high particle loads (>108 cells/mL) in an economical way. Deciding on the right purification system involves addressing questions about process performance,…
Use of Dynamic Control in Periodic Counter-Current Chromatography
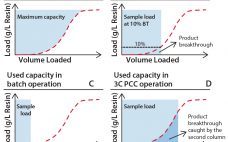
Interest in continuous bioprocessing is growing rapidly, and such an approach has shown to have a potential in providing gains in both productivity and savings in cost of goods sold. The ÄKTAâ„¢ pcc 75 chromatography system can be used for purification of target proteins in continuous downstream processes using periodic counter-current chromatography (PCC). PCC enables greater use of chromatography resin capacity, allowing sample loading to much higher levels than what is possible in traditional batch chromatography by using safety factors…
Designing a MAb Purification Process: Based on Bio-Sequential Chromatography
In batch-mode chromatography, operating conditions do not allow full use of the total media capacity (1, 2). Bio-Sequential Chromatography (BioSC®) is an open-loop process for the separation of multicomponent solutions (3). The unique column of the batch mode is replaced by a series of smaller columns (from two to six). This design maximizes the use of the media without product loss along the process sequences. The BioSC® Lab automated system is based on the multicolumn chromatography process, designed and provided…
Adsorption Study of Polymer-Modified Cellufine CEX Resin
Cellulose is well known as a natural raw material that has mechanical strength, lower nonspecific adsorption, and good biocompatibility. In addition, cellulose particles have unique pore-size characteristics appropriate for chromatography of biopharmaceuticals. Ion-exchange chromatography (IEX) is an important step for biopharmaceutical manufacturing. Specifically, cation-exchange chromatography (CEX) can be used as a capture step in monoclonal antibody (MAb) purification. Recently, advanced IEX resins have been developed using polymer modification techniques. Initial screening of conditions such as pH and ionic strength is…
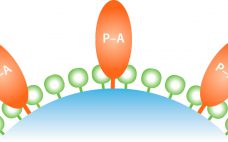
Platform Purification of Six Biosimilar Molecules Using Amsphere A3 Protein A Resin
Currently more than 70 biosimilar monoclonal antibodies (MAbs) are under development, and multiple originator MAbs are going off patent in the next three to four years. Protein A affinity media material remains the most important workhorse for the purification of monoclonal antibodies. Protein A media has a high impact on both development and manufacturing costs, particularly during early stage clinical phases. This note summarizes key performance parameters for JSR Life Science’s Amsphere A3 high-capacity protein A resin for six biosimilar…
Ultrasonic Technology: Single-Use High-Precision Flowmeter
Levitronix® has released the first ultrasonic, single-use (SU) flowmeter on the market. Characterized by high accuracy and superior performance, LEVIFLOW® SU noninvasive flowmeters enable a highly precise and low-cost flow measurement. With an accuracy of <1% of reading and flow ranges from 1 mL/min to 80 L/min, they offer the highest level of accuracy for measuring liquids in biopharmaceutical and other applications that require FDA, USP-VI, BSE/TSE, and animal-component–free materials that can be gamma sterilized. Technical Background: Figure 1 illustrates the…
Buffer In-Line Dilution and LPLC: Analytical Accuracy with LEWA EcoPrime LPLC Systems
The new LEWA EcoPrime® LPLC chromatography and enhanced buffer dilution platform combines proprietary fluid design and the LEWA Intellidrive® pump technology to deliver unrivaled reproducibility and accuracy. The integrated buffer in-line dilution (BID) option prepares point-of-use buffers combining two unit operations into a single, space-saving skid. A single LEWA EcoPrime system has the flow range of up to two conventional LPLC units while delivering gradient accuracy of greater than ±1%. Because of its dynamic range, EcoPrime LPLC can bridge the gap…
Integrated and Scalable Continuous Chromatography: The Cadence BioSMB PD System from Pall
Bioprocess intensification leads to more efficient, flexible, and cost-effective biopharmaceutical manufacturing facilities. Single-use systems designed for continuous operation facilitate process intensification. Pall Life Science’s Cadenceâ„¢ BioSMB PD platform is the first disposable flow path, continuous multicolumn chromatography solution that is fully scalable from a process development laboratory to good manufacturing practice (GMP) manufacturing. It provides an economically viable, disposable chromatographic solution for even the most demanding and costly chromatographic steps. During multicolumn countercurrent (also known as simulated moving bed, or…
Single-Use Systems: Balancing the Need Between Customization and Standardization
In June 2016, Parker domnick hunter began manufacturing in its new single-use facility in Birtley, Co Durham, UK. Here I discuss the approach taken in the design, testing, and manufacturing of single-use assemblies and some of the advantages they can have in terms of lead times, inventory control, and supply chain robustness. Challenges of Customization For some time, the message relayed to biopharmaceutical manufacturers was to design exactly what you want and need for each process. That meant a customized…