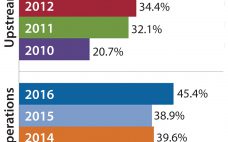
In 2012, to mark BPI’s 10th year of publication, we launched our biennial BioProcess International Awards program. Our publication has from the start aimed to help drive advancements in this industry, not simply to report on them. We have to date (in 2012, 2014, and now 2016) recognized more than 50 people and companies that represent excellence in leadership, corporate citizenship, collaborations and partnerships, and innovative technologies and applications. This year, our 16-member judging panel evaluated each nomination within assigned…
November 2016
Spotlight for November
EU Workshop Report on Authorization of Advanced Therapy Medicinal Products The European Medicines Agency (EMA) published a report from a multi-stakeholder expert meeting held in May exploring ways to foster the development of ATMPs in Europe and expand patients’ access to these new treatments. ATMPs comprise gene therapies, tissue-engineered products, and somatic cell therapies. These medicines have the potential to reshape treatments of a wide range of conditions, particularly in diseases for which conventional approaches are inadequate. However, eight years…
Outsourcing of Buffer Preparation Activity Is Increasing
The major fluid products used in bioprocessing — culture media and buffers — are classically prepared in-house by rehydrating (dissolving and mixing) powders purchased from suppliers. Most bioprocessing facilities consider in-house preparation of these fluids to be a core bioprocessing task. However, some companies are outsourcing the work either by purchasing preprepared materials from vendors or hiring contract manufacturing organizations (CMOs) to prepare them. Buffer fluid preparation is one area of downstream production operations that are seeing an increase in…
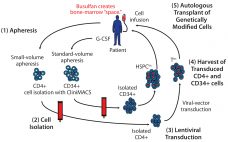
Cell-Delivered Gene Therapy: This Viral Vector Manufacturing Method Could Widen Its Applicability
Cell-delivered gene therapy is making an impact on a range of diseases (1–17). To date, successful treatments have generally been in conditions involving genetic deficiencies/abnormalities, for which introduction of a normal gene allele has been corrective (1–12, 18). Such an approach requires a vector containing the normal allele to overcome the mutant or lacking gene. The vector of choice for cell-delivered gene therapy is often a lentivirus that integrates and expresses introduced therapeutic genes in host target cells and their…
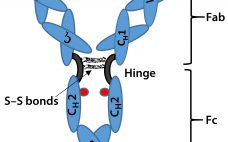
Biosimilar Therapeutic Monoclonal Antibodies: Gaps in Science Limit Development of an Industry Standard for Their Regulatory Approval, Part 2
Last month, Part 1 of this discussion briefly described the regulatory landscape for developing biosimilar therapeutic monoclonal antibodies (TMAbs). We identified certain specific structural components of TMAb drug substances that warrant particular attention because alterations to them are likely to affect therapeutic safety and effectiveness. Now we conclude by considering whether studies of reference materials can further the development of analytical industry standards to ensure comparability of putative biosimilar TMAbs with innovator TMAbs. We suggest that the time is right…
Special Report on Antibody-Drug Conjugates: Technical Challenges and Opportunities
Among the emerging targeted therapies in biotechnology, antibody–drug conjugates (ADCs) hold a unique position. An ADC consists of a monoclonal antibody (MAb) with affinity to tumor cells, a cytotoxic small-molecule payload, and a linker connecting the two. Together the MAb, conjugation chemistry, and cytotoxin increase the complexity of ADCs several-fold relative to unmodified MAbs — and exponentially relative to chemotherapies. Viewing ADCs as hybrids of antibody- and chemotherapy-based cancer therapies is tempting. That description applies chemically and structurally, but ADCs’…
Engineering Tissues with Bioprinting
Commonly referred to as three-dimensional (3D) printing, additive manufacturing encompasses a set of technologies that fabricate objects in an additive way, layer by layer, rather than conventional means of fabrications that generally subtract unwanted material from a larger block. Precise control over material placement allows 3D printing to fabricate objects that otherwise would not be manufacturable. Although many of these technologies have been around for two or three decades, recently they have received a significant amount of attention from industry,…
Mass Spectrometric Conjugate Characterization: Process Qualification of Recombinant Protein–Hapten Conjugation
Conjugated protein biotherapeutics such as PEGylated proteins (with polyethylene glycol), antibody–drug conjugates (ADCs), and protein–haptens often present unique analytical challenges related to characterizing the conjugation aspect of their manufacturing processes. Analytical characterization of this class of proteins requires knowledge of the sites of conjugation, the degree of conjugation, and the drug-to-protein ratio. Here we present case studies in development of reliable methods based on mass spectrometry (MS) to characterize a protein–hapten drug substance during late-phase process validation. This protein is…
Providing Lipids Boosts Protein Productivity: Testing a Feed Supplement with Multiple Cell Clones and Media Formulations
As the biologics (and now biosimilar) markets continue to grow, pressure increases on biomanufacturers to reduce cost of goods sold (CoGS). One way they can reduce cost is by increasing protein productivity in terms of protein titer per volume of culture. Media optimization is a key strategy for increasing protein productivity. In the past few decades, average titers across the industry have increased greatly — from <0.5 g/L in the 1980s to >3 g/L today, and it is not uncommon…
Advanced Protein Engineering Enhances Biopharmaceutical Manufacturing and Analytics
Production of proteins for pharmaceutical use is a complex, multistep process that requires technologies for purifying such molecules from highly complex biological mixtures. It also calls for reliable, cost-effective, high-throughput analytical techniques to determine protein quality and functionality to ensure the safety and efficacy of end-products. Mistakes in product development and manufacturing not only are immensely costly, but they can also put patients at risk. Many well-established processes and analytical tools are available for use in manufacturing antibody drugs (e.g.,…