Increased understanding of human diseases at molecular and cellular levels is leading to development of novel life-science technologies. Such advancements typically pertain to discovery and manufacturing of novel human therapeutics, new modes of drug delivery, and novel diagnostic technologies. The majority of those technologies are developed by early stage biopharmaceutical companies that have a greater appetite for risk than do larger companies. Early stage biopharmaceutical companies, however, have limited capital raised through personal sources, angel investors, venture capital, or government…
June 2014
Industry Experts Convene in New York to Discuss Latest Innovations: A BPI Special Report
As the biopharmaceutical industry continues to mature and grow, so too does the need to educate a broader audience of biopharmaceutical professionals interested in hearing, understanding, and applying the latest science and technology trends that support and in many cases are transforming today’s bioprocesses. To reach this extended and engaged audience, BioProcess International created the BPI Theater Series: a live, interactive program that provides bioprocessing content to traditional, noncore biopharmaceutical conference programs. It provides attendees with the opportunity to interact…
Higher-Order Structure Comparability: Case Studies of Biosimilar Monoclonal Antibodies
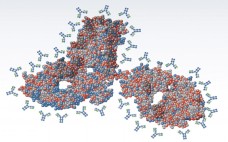
Great successes for monoclonal antibody (MAb)–based biologics over the past decade have provided many valuable options for patients combating some of the most serious diseases in the world, including cancer and autoimmune diseases. MAbs and antibody–drug conjugates (ADCs) are among the fastest growing biologic segments in development, with hundreds of candidates currently under clinical study. Meanwhile, society is facing the challenge of increasingly higher costs in healthcare including the cost of pharmaceuticals. With an aging population in many parts of…
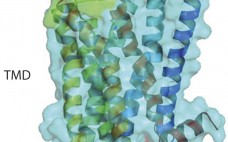
Targeting G Protein–Coupled Receptors with Biologics for Therapeutic Use, Part 1
G -protein coupled receptors (GPCRs) represent a target superfamily linked to many disorders across all therapeutic areas. Although this target class has been historically treated by small molecules and peptides, antibodies can offer a number of advantages over such molecules by virtue of their specificity, dosing frequency, and restricted penetration. They also can provide other functional effects specifically mediated by the Fc region (ADCC and CDC) as well as different modalities such as those offered by bispecific and antibody drug…
Site-Specific Characterization of Glycosylation on Protein Drugs
A large proportion of biotherapeutic products are glycoproteins. These include erythropoietin and other cytokines, antibodies, glycosyltransferases, and glycosidases, which together generate billions of dollars in sales worldwide. Such drugs are inherently complex. As new treatments emerge and biosimilars are evaluated, the need to better understand their molecular structures is more acute than ever. Therapeutic glycoproteins are typically produced as recombinant products in cell culture systems. Glycosylation is of major importance during development of these drugs because their glycan chains markedly…
Qualification of Scale-Down Bioreactors: Validation of Process Changes in Commercial Production of Animal-Cell-Derived Products, Part 2 — Application
Here we apply our approach to validation of animal cell culture process changes using qualified, scale-down bioreactors. As described in Part 1 (including Table 1, Figures 1 and 2, and References 1–23), the goal is to facilitate implementation for the benefit of both the patients and industry. “Qualification of Scale-Down Bioreactors: Validation of Process Changes in Commercial Production of Animal-Cell–Derived Products, Part 1 — Concept” appears on pages 38–45 of BioProcess International’s May 2014 issue. Process changes often entail validation,…
Enabling Greater Process Control and Higher Protein Titers: Advances in Downstream Single-Use Technologies
Downstream protein purification (the stage in which a protein is isolated and purified) is one of the last steps in biotherapeutic manufacturing. Single-use technologies are an increasingly popular choice for both upstream and downstream bioprocessing because they offer significant benefits over traditional multiuse manufacturing systems. Single-use technologies also provide an array of logistical benefits, including reduced costs, minimized risk of cross-contamination, and improved operational efficiency (1). Challenges remain, however, in designing a complete, streamlined, single-use process for downstream protein purification.…
One Billion Mesenchymal Stem Cells in an Eppendorf BioBLU 5c Single-Use Bioreactor at 3.75-L Scale
For BPI’s inaugural “Ask the Expert” webcast, Ma Sha (Eppendorf’s director of technical applications) fielded questions related to his upcoming poster presentation at IBC’s Single-Use Applications for Biopharmaceutical Manufacturing in Boston this month: “One Billion Mesenchymal Stem Cells in Eppendorf BioBLU 5c Single-Use Bioreactor 3.75-L Scale”. Eppendorf R&D Labs is formerly New Brunswick Scientific, which was acquired by Eppendorf in 2007. Sha’s Presentation Our focus recently had been large-scale stem-cell applications in bioreactors. We chose to work on mesenchymal stem…
Transformative Healthcare: The National Biomarker Development Alliance
A newly launched independent, nonprofit organization — the National Biomarker Development Alliance (NBDA) — will broadly engage leaders in industry, academia, patient groups, and government from across the United States. It was announced on 13 January 2013 at the National Press Club by the Research Collaboratory (RCASU) at Arizona State University (ASU). The mission of the NBDA is to address the complex and urgent challenge of creating standards needed for end-to-end, systems-based biomarker research and development (R&D). The alliance is…
June 2014 Issue Author Insights
Exclusive author commentary and article previews for the BioProcess International June 2014 issue.