Complex drug development and biomanufacturing processes involve back-and-forth shuttling of activities among multiple functions. Close communication, collaboration, and coordination among stakeholder departments and functions are needed to successfully execute these processes. Whereas collaboration between multiple functions leverages each function’s expertise, the resultant structure also poses several challenges, as listed in Table 1 (1). These challenges are further exacerbated as an organization grows in size and geography (2, 3).
In the absence of clarity and appropriate assignment of roles and responsibilities, organizations may end up wasting time and resources. To address potential challenges of cross-functional processes, the following must be taken into account from an operational perspective (4):
- Roles and responsibilities of individual groups must be clarified.
- Each activity needs to have an assigned owner.
- Redundant or non–value-adding activities must be eliminated. Each activity introduces an additional burden on time and resources, so existence of each activity must be justified.
Process effectiveness analysis can address those challenges by breaking down the processes into individual activities and analyzing how they “interact” with each other. Process mapping is a tool that can facilitate process effectiveness analysis. Process mapping tools such as flowcharts and process diagrams are routinely used for project management. Here, we demonstrate how process effectiveness analysis can address process gaps and redundancies, and streamline processes. SMC recommends that all biopharmaceutical companies perform process effectiveness analysis for operational efficiency.
API AnalytIcal TestIng and Release Process
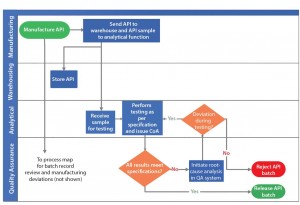
Figure 1 depicts an active pharmaceutical ingredient (API) analytical testing and release process as a process map. This example does not represent a process at a specific organization and is provided for illustration only. Using it, we discuss the following aspects of process effectiveness analysis:
- Clarify ownership of activities by function, close process gaps, and eliminate redundancies
- Streamline processes by eliminating noncore activities and simplifying communications.
Clarify Ownership of Activities and Close Process Gaps
Figure 1 shows activities from API manufacture to release of API batches. Activity owners are listed alongside each activity. Identifying activity owners clarifies the roles and responsibilities of each function. Each activity is associated with predecessor and successor activities. The process map clarifies the material and information flow that must occur at each step of an API testing and release process. Process maps analysis helps identify critical process gaps such as unavailability of a required material or information needed to appropriately perform each activity. Process maps also help identify redundant activities that must be eliminated. The scenario in Figure 2 shows how process gaps and redundancies were identified and addressed, as described below.
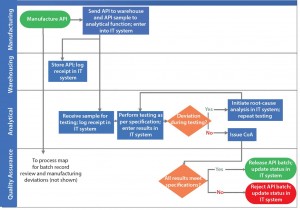
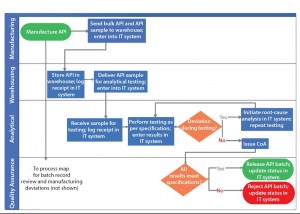
Figure 2: Depiction of a scenario of addressing process gap and process redundancy using example in Figure 1
Process Gaps: Figure 1 includes no systems for information transfer and hand-off. Through suitable information technology (IT) systems (Figure 2), the organization can ensure that information will be transferred consistently. That enables each subsequent activity to occur as desired as well as traceability of good manufacturing practice (GMP) operations.
Process Redundancies: As Figure 1 shows, root-cause analysis is initiated by quality assurance personnel only if a result does not meet specification. If a specification is not met because of a deviation during testing, then multiple certificates of analysis (CoAs) will be generated for the same API lot. By contrast, suitable controls during analytical testing can detect deviations during API testing, irrespective of the testing result meeting the specification. That also will increase compliance of the testing process through investigation of all deviations and will eliminate redundancy of issuing multiple CoAs (Figure 2).
Streamlining Processes for Increased Efficiency
Processes are networks of activities. As the number of network nodes increases, the amount of time and number of resource requirements also increase. Process effectiveness analysis allows organizations to improve operational efficiency in critical and core activities, communications, and cross-functional decision points.
Critical or Noncritical, Core or Noncore Activities: Noncritical activities may be eliminated, outsourced, or restructured. Similarly, the number of noncore activities must be reduced for each department. Networks should minimize the number of nodes by focusing on critical and core activities.
Complexity of Communications: A process in which multiple activities feed into a single activity or a single activity results in multiple activities increases complexity of communications. Such processes must be monitored closely. Organizations must streamline the communications and derisk those activities.
Cross-Functional Decision Points: Decision points at which a cross- functional team needs to make decisions specifically require the focus of the organization to ensure success of that process. Using the previous example, Figure 3 shows how process effectiveness analysis can streamline processes by eliminating noncore activities and simplifying communications.
In Figure 2, API manufacturing results in two successor activities: bulk API transfer to a warehouse and API sample to an analytical function. By making the warehouse function responsible for providing the API sample to the analytical function, all network nodes in the process are single-input–single-output, thereby simplifying the communications required to execute this process. In addition, the proposed process change eliminates manufacturing’s noncore activity of material transfer and leverages the warehouse’s core activity of material transfer.
Critical Process Analysis
Biopharmaceutical processes must be carefully analyzed and monitored. Roles and responsibilities within a company must be clear, and processes must be streamlined for increased operational efficiency. Streamlining processes becomes even more valuable when multiple independent processes intersect with each other on an enterprise level. So it is important that all biopharmaceutical companies — from early stage startups to large manufacturers — perform process effectiveness analysis on an enterprise level for operational efficiency. Doing so will shorten development timelines, lower development and operating costs, and create sustainable growth of an organization. Process monitoring and analysis are continuous activities that are critical to success of an organization and should be integral to a company’s strategy.
RefeRences
1 Jassawalla AR, Sashittal HC. Building Collaborative Cross-Functional New Product Teams. Academy of Management Executive 13(3) 1999: 50–63.
2 Gossieaux F. Enabling Collaboration in the Pharmaceutical Industry. BioPharm Intl. June, 2002: 105–106.
3 Zedtwitz MV, Gassmann O, Boutellier R. Organizing Global R&D: Challenges and Dilemmas. J. Int. Management 10, 2004: 21–49.
4 Snyder R. Aligning for Breakthrough Results. Breakthrough, Inc., 2008 (online); www.breakthrough-inc.com/typeroom/assets/ uploads/media/Cross_Functional_Alignment_ Case_Study.pdf.
Siddhartha (Sid) Jain is a managing partner at SMC Consulting Group LLC, 404 Brunswick Drive, Troy, NY 12180; sid.jain@ smcstrategy.com. For any questions regarding this article or to learn about SMC’s services, visit our website at http:// www.smcstrategy.com.