Single-use bioreactor maker PBS Biotech has closed a private $10 million funding round with BroadOak Capital Partners to advance its Vertical-Wheel technology.
The funding will be used to expand PBS’ internal operations and capacity, the firm told Bioprocess Insider, to meet the demand and need for its biomanufacturing customers, specifically for cell therapies.
Such “investment in manufacturing, engineering and contract services capabilities is required to maintain our rapid growth trajectory in the cell therapy market, we were told.
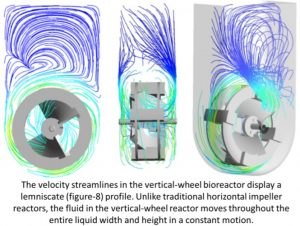
Image taken from PBS poster: Borys et al., Computational Fluid Dynamics (CFD) Modeling of Single-Use, Vertical-Wheel Bioreactors as a Predictive Scale-up Tool for Large Scale Stem Cell Culture
The California-headquartered company’s products are based on its Vertical-Wheel technology, which it says is superior to traditional stirred-tank bioreactor for the production of therapeutics.
“Currently available single-use bioreactors, such as those with rapidly spinning horizontal impellers, create a heterogeneous liquid mixing environment. Significant differences in hydrodynamic conditions at larger bioreactor volumes compared to small volumes present a challenge in predicting cell culture performance during process development scale up,” the firm said.
“In contrast, the combination of Vertical-Wheel impeller and U-shaped vessel uniquely creates a homogeneous liquid mixing environment. Similar hydrodynamic conditions can be achieved at different volumes of Vertical-Wheel bioreactors, enabling the development of truly scalable cell culture processes.”
PBS said the tech is particularly suited for advanced therapy makers as scalable and reliable single-use bioreactor technology will be critical for commercial production of high-quality allogeneic cell therapies.
Cell therapy products, typically being anchorage-dependent cells that grow on the surface of microcarriers or as cell aggregates, are sensitive to a bioreactor’s hydrodynamic conditions the firm told us.
“Vertical-Wheel bioreactors can fully suspend and continuously agitate large particles such as microcarriers or aggregates in homogeneous and scalable hydrodynamic conditions.
“Scalable and reliable single-use bioreactor technology will be critical for commercial production of high-quality allogeneic cell therapies.”
