The increased capabilities in Singapore will support both upstream and downstream processing and analytical development, the CDMO says.
The size of the investment has not been disclosed, but Jeetendra Vaghjiani, senior director of Clinical Development for Mammalian Biologics, Lonza told this publication the expansion consists of a 1800 m2 lab built at the Singapore Science Park in Tuas along with the renovation of an existing 1500 m2 lab.
“We are adding more capacity across all our technologies, supporting upstream and downstream processing and analytical development,” he said.

Lonza’s site in Tuas, Singapore
“This includes more than doubling our laboratory-scale and miniaturized bioreactor capacity and adding the capability for protein characterization by mass spectrometry for the first time in Singapore.”
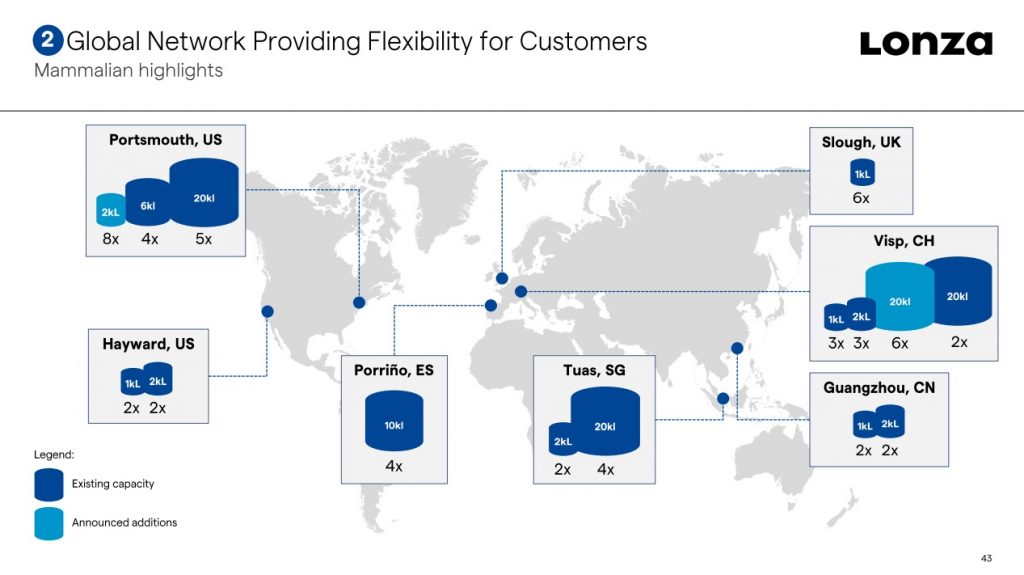
The Swiss contract development and manufacturing organization (CDMO) laid plans for its Singapore biologics facility in 2006, building a plant boasting 4 x 20,000 L stainless steel bioreactors. Since then, the firm has steadily invested to increase both capacity and service offering from the site, and in 2016 added multiple 2,000 L single-use bioreactors.
“We constantly assess our assets and invest in developing and installing new capacity and technology to meet the market demand in biologics,” said Vaghjiani. “As we have expanded our manufacturing footprint, this has increased the need for development services to support those manufacturing slots.”
The new lab started operations last month while the renovation is set to be completed by the end of the year.
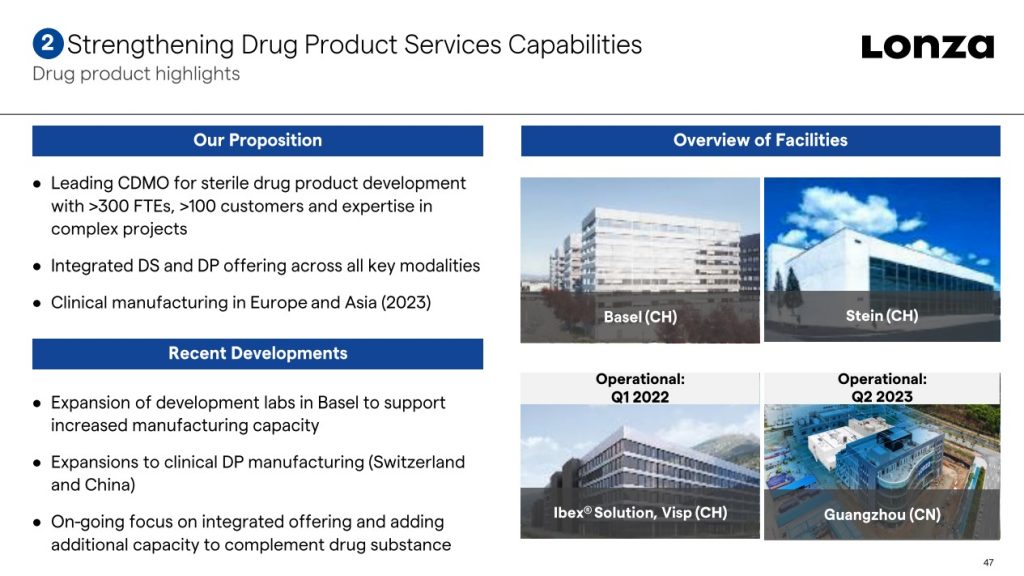
The expansion forms part of an ambitious investment program by the CDMO in its biologics business to address high demand for biomanufacturing. As well as announcing a plan to invest $935 million into its sites in Visp, Switzerland and Portsmouth, New Hampshire earlier this year, Lonza has also begun expanding drug product capabilities in Switzerland and China.
The firm highlighted its drug substance and drug product capabilities at its 2021 Capital Markets Day earlier this week: