Biomanufacturers seeking the best approach to rapid implementation of flexible manufacturing capacity take into account the benefits presented by different modular construction options. We analyzed different approaches to building manufacturing capacity and assessed the economic benefits of each approach. Our evaluation was based on biopharmaceutical products for which there is an immediate unmet need, such as treatments or vaccinations for COVID-19. Such products also might entail a sudden increase in demand (e.g., expansion of a product indication or sales ramp…
Author Archives: Yuki Abe
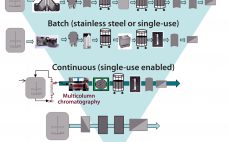
Standardized Economic Cost Modeling for Next-Generation MAb Production
Historically, in generating material for clinical testing during antibody process development, emphasis was placed on efficacy, product quality, regulatory compliance, and speed. As the biopharmaceutical industry has matured (and with increasing competition), emphasis has shifted toward cost optimization and manufacturability. Reducing the costs of medicines for patients and payers (thereby broadening access to drugs) is now a key driver during development of new therapies as well as modernizing processes for existing molecules. Cost reduction includes providing robust manufacturing processes that…