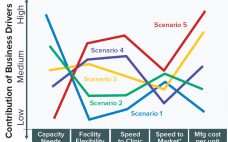
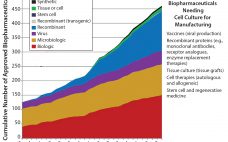
Drug Substance Scenarios Given the complexity of the biopharmaceutical industry and the increasing diversity of products and companies, it is clear that there will be no “one size fits all” solution to biomanufacturing. Instead, we see a range of biomanufacturing scenarios playing out over the next 10 years. Five high-level scenarios were selected for drug substance manufacturing and two for drug product to cover the full spectrum of process and facility types. Each facility type is associated with a representative…
Author Archives: Steve Jones
BioPhorum Operations Group Technology Roadmapping, Part 2: Efficiency, Modularity, and Flexibility As Hallmarks for Future Key Technologies
For a complex biopharmaceutical industry, setting out to forecast future technologies must involve considering how such technologies will be used. In the first article (1), I discussed why there was a need to develop a technology roadmap for the biopharmaceutical industry and the trends shaping its future: namely, the introduction of new product classes, the continued growth of the biopharmaceutical market, pressure to reduce costs, and uncertainty in approval and sales of new products. Herein I discuss the technology roadmap’s…
BioPhorum Operations Group Technology Roadmap, Part 1: Four Trends Shaping the Future of the Industry
What prompts over 100 biopharmaceutical manufacturers, leading academics, supply partner R&D heads, regulators, and worldwide regional hubs to get involved in a major project? It’s when that project identifies the future technology needs of the biopharmaceutical manufacturing industry and accelerates its collective innovation. In February 2015, the BioPhorum Operations Group (BPOG) Technology Roadmapping steering committee met in Washington DC to create the first “technology roadmap” for the biopharmaceutical manufacturing industry. The biopharmaceutical market has been experiencing dramatic changes: explosive growth…