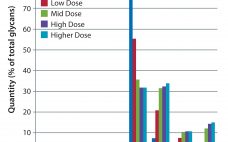
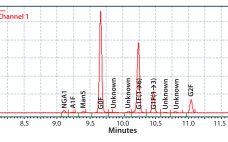
In Part 1 of this report, we described our development of a high-throughput assay for analyzing monoclonal antibody (MAb) glycans and how we used it to evaluate the effects of medium supplements on galactosylation of MAbs produced by two different cell lines (1). This month, we examine galactosylation of a MAb produced by a third cell line. A discussion follows on the benefits of this high-throughput assay before we highlight the similarities and differences in galactosylation among the three MAbs…
Author Archives: Sadettin Ozturk
Enhanced Galactosylation of Monoclonal Antibodies: Using Medium Supplements and Precursors of UDP-Galactose, Part 1
The biopharmaceutical industry needs better understanding of how monoclonal antibody (MAb) glycosylation is influenced by components in cultivation media — and it needs methods to exert some control over the structure of MAb glycans. That structure can affect MAb function. Thus, a high-throughput (HTP) assay is needed for characterizing MAb glycosylation so that developers can observe the effects of cultivation conditions on MAb glycosylation rapidly, with a goal of producing MAbs that have a desired glycan structure. The method also…
Development of a High-Throughput Formulation Screening Platform for Monoclonal Antibodies
The goal of formulation development for therapeutic proteins is to find conditions under which a protein remains stable during storage, transport, and delivery to patients. Both chemical and physical stability must be considered. Chemical stability is related to the rates of chemical modification to a protein molecule such as deamidation of aspargine residues and oxidation of methionine residues (1, 2). Particularly important to control if they affect biological function, those modifications could also lead to changes in conformation or half-life…