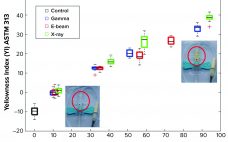
Ionizing-technology–based industries are growing rapidly around the world. The expansion is driven mostly by the technology’s myriad applications, including polymer crosslinking, medical device sterilization, food pasteurization, and phytosanitary treatment. Ionization also is used in the manufacture of some healthcare products such as medical devices and biopharmaceuticals. Industrial sterilization methods render single-use products and manufacturing components safe and ready for their intended use. ISO11137-1 describes the validation and routine control of a sterilization process for medical devices and mentions the three…
Author Archives: Samuel Dorey
Development of a Standardized Extractables Approach for Single-Use Components: General Considerations and Practical Aspects — A Manufacturer’s Perspective
The subject of extractables for single-use bioprocess contact materials has been a subject of heated debate since roughly the summer of 2012, when the first ISPE paper was published issuing a call to action to develop a standardized extractables protocol for the industry (1). As a supplier that pioneered the science of extractables (2‒11) and has published extractables data for our products for over 20 years, Sartorius Stedim Biotech (SSB) took the opportunity to look back, take stock, rationalize, and…