A new product development approach will be described here for the affinity capture of monoclonal antibodies (MAbs) from cell culture materials using a novel base stable rProtein A ligand with up to six available Fc binding sites. The resin is based on a stable cellulose bead structure with excellent flow properties combined with the affinity ligand immobilized at multiple sites to yield a robust next generation MAb capture resin with a high level of binding capacity. These resins have been…
Author Archives: Shigeyuki Aoyama
Development of a Next Generation Cellulose-Based High Capacity rProtein A Capture Resin for High Throughput MAb Purification in Both Batch and Continuous Purification Formats
A new product development approach will be described for the affinity capture of Mab’s from cell culture materials employing a novel base stable rProtein A ligand. Using a stable cellulose base bead with excellent flow properties coupled with a novel immobilization methodology, a next generation rProtein A capture resin has been developed with a high level of antibody binding capacity. The new Cellufine™ rProtein A resin shows C20% dynamic binding capacity (DBC) of >50 mg/ mL with polyclonal antibodies at…
A Novel Cellufine Mixed-Mode Resin: Cellufineâ„¢ MAX IB for Polishing of Monoclonal Antibodies
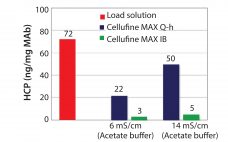
Cellulose is well-known as a natural raw material that has mechanical strength, lower nonspecific adsorption, and good biocompatibility. Additionally, cellulose particles have unique pore-size characteristics appropriate for the chromatography of biopharmaceuticals. Mixed-mode chromatography resins are well known to have unique selectivity differences from traditional IEX or HIC resins. Cellufineâ„¢ MAX IB, a novel cellulose-based mixed-mode resin, has polyallyl amine partially modified with butyl groups ligand (Figure 1). The resin is used in flow-through mode after a protein A step in…
Adsorption Study of Polymer-Modified Cellufine CEX Resin
Cellulose is well known as a natural raw material that has mechanical strength, lower nonspecific adsorption, and good biocompatibility. In addition, cellulose particles have unique pore-size characteristics appropriate for chromatography of biopharmaceuticals. Ion-exchange chromatography (IEX) is an important step for biopharmaceutical manufacturing. Specifically, cation-exchange chromatography (CEX) can be used as a capture step in monoclonal antibody (MAb) purification. Recently, advanced IEX resins have been developed using polymer modification techniques. Initial screening of conditions such as pH and ionic strength is…
Adsorption Study of Egg-Derived Influenza Virus with Cellufine Sulfate Affinity Chromatography Media
An efficient process for purifying virus particles is important when developing a vaccine. Cellufine Sulfate affinity chromatography medium has been used for manufacturing viral vaccines such as influenza virus, rabies virus, and Japanese encephalitis virus. Here we describe how to purify egg-derived influenza with Cellufine Sulfate media. Figure 1 shows a typical chromatogram of inactivated influenza virus A strain (H7N7) from allantoic fluid with Cellufine Sulfate media. Table 1 shows that adsorbed virus particles are eluted from the medium easily…
Removal of Antibody Aggregates Using Cellufine MAX GS
Purification of therapeutic monoclonal antibodies (MAbs) requires advanced and selective means for separating aggregates from target monomers. Cellufine MAX GS chromatography media are optimized for higher capacity, better separation, and greater selectivity to improve downstream purification of MAbs. JNC Corporation’s new strong cation exchanger, Cellufine MAX GS, has optimized ligand density for improved dynamic binding capacity and selectivity and superior pressure flow characteristics, which are required by MAb purification platforms. MAb Monomer/Aggregates Separation: Cellufine MAX GS exhibits exceptional selectivity between…