The merging of Informa‚Äôs KNect365 division with pharmaceutical events operated by UBM brings another opportunity for BPI‚Äôs successful ‚ÄúBPI Theater‚ÄĚ program: the ‚ÄúBioLIVE‚ÄĚ theater taking place at CPhI. Our first foray into the theaters ‚ÄĒ our own speaker/roundtable sessions held in the exhibit halls of large events ‚ÄĒ was at a BIO International Convention some years ago. Through our relationship with the Biotechnology Innovation Organization, that came about to bring more technical content into the big event. As BIO grew…
Author Archives: S. Anne Montgomery
Continuous Chromatography: Experts Weigh in on the Possibilities and the Reality
Discussions of continuous processing in the biopharmaceutical industry are an important part of current efforts toward intensifying bioproduction and bioprocessing. Biomanufacturers are looking at all components of their development and manufacturing processes for ways to reduce the size of their facilities, lower costs, and increase speed and flexibility of operations. Increasing options for and availability of single-use technologies have been major enablers of myriad attempts to improve efficiencies. Although the general consensus may still be that single-use components are more…
Manufacturing Insights: Interviews from BWB 2018 Highlight Perspectives on Streamlining Manufacturing Timelines
Addressing manufacturing and technologies strategies to accelerate market entry is one of BPI‚Äôs highlighted themes for 2019. In partnership with our conference colleagues in Informa‚Äôs KNect365 division, this already has been a shared theme, reflecting the general goals of the industry and related advice from its regulators. BPI‚Äôs summer 2018 preconference ebook included interviews by Dan Stanton (editor, BioProcess Insider) with speakers previewing their talks for the BPI Conference in Boston on 7 September 2018. Two of those conversations focused…
January-February From the Editor
Greetings from the BPI editorial staff ‚ÄĒ we wish you a healthy and prosperous 2019! Beginning with a combined January‚ÄďFebruary issue somehow makes the year seem to be speeding by right from the start. We are immersed now in the first radical redesign of our magazine since the publication‚Äôs founding in 2003. Some aspects proceed smoothly; others, not so much. You see with this issue a new cover design that ties us in with our KNect365 event brands. You‚Äôll see…
Messenger RNA Drugs: Engaging the Machinery of Patients’ Cells to Therapeutic Effect
Although most of the bioprocess industry has focused on process development for large-molecule formulations (e.g., protein drugs), a growing segment of the industry has been concentrated on other types of biotherapeutics to leverage advances in understanding of immunology and genetic engineering. Such technologies may emerge both as tools for drug manufacturing and at some point, as biopharmaceuticals, biotherapeutics, vaccines, and cell and gene therapies, ¬†themselves. What brings mRNA research to BioProcess International‚Äôs attention is the increasing interest turned toward therapeutic…
Introduction: The Ins and Outs of Market Demand
From transport and holding of bulk drug substance to shipping, warehousing, tracking, and distribution of final packaged drug products, biopharmaceutical supply chain logistics can be described as an industry in itself. And that‚Äôs just one side of the story. Even though much of the work of establishing and maintaining supply chains might happen outside the manufacturing environment, all organizations that develop processes and final products depend on having the raw materials and available components and resources to do their work.…
Rolling with the ‚ÄėTides: Elucidating the Role of Peptides and Oligonucleotides in the Biopharmaceutical Industry
In earlier issues of BPI we published a few ‚ÄúElucidation‚ÄĚ closers that we called ‚ÄúDefining Moments.‚ÄĚ Since then, we have tried to distinguish key confusable terms from one another. Those presented (and sometimes ‚Äúelucidated‚ÄĚ) have been analytical and bioanalytical, spectroscopy and spectrometry, and biosimilars and biobetters. They are just a few of the many confusable terms in the biopharmaceutical industry. For example, when someone says ‚Äúdrug delivery,‚ÄĚ a formulator will think of a syringe or transdermal patch, but a logistics…
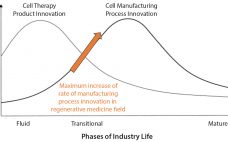
The Need for Adherent Cell Manufacturing: Production Platform and Media Strategies Drive Cell Production Economics
Most commercial biopharmaceuticals originated from academic research laboratories and start-up development laboratories. Despite such products having differences in modalities and targeted disease indications, and whether their target patient populations are relatively small or approaching blockbuster status, at a key point in development, biopharmaceutical production must scale up from laboratory to commercial production. That movement from research to development and then to manufacturing forces attention on economics and speed to market, and it drives innovative approaches to producing biopharmaceutical cell compositions…
October From the Editor
As we send this issue to the printer, the BPI editors are enjoying the start of the western Oregon fall season. Early rains are beginning finally to extinguish the remaining wildfires that have kept our valley (along with much of the west coast) choked with smoke much of the summer. With the fires on this side of the country and the recent storms to the east, we hope that all our colleagues and readers remain safe. For BPI, changes begun…
Big Biotech Data: Implementing Large-Scale Data Processing and Analysis for Bioprocessing
Managing large amounts of data presents biopharmaceutical companies of all sizes with the need to adopt more efficient ways to handle the ongoing influx of information. At KNect365‚Äôs September 2017 Cell and Gene Therapy conference in Boston, Lisa Graham (founder and chief executive officer of Alkemy Innovation, Inc. in Bend, OR) spoke about the need for data management, data analysis, and process monitoring systems to evolve. Although she was speaking at a cell therapy event, her points are applicable to…