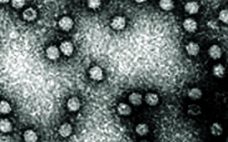
Viral safety is a critical focus during biopharmaceutical manufacturing (1–5). Although well-characterized mammalian cells such as the Chinese hamster ovary (CHO) line have been used for decades, both endogenous expression of retroviral-like particles and exogenous contamination events from viruses warrant continued vigilance (6, 7). International regulatory agencies require biomanufacturers to validate the “viral clearance” efficacy of their downstream manufacturing process steps before resulting products can be awarded clinical trial or commercial approval (8–10). Currently, viral clearance testing is based on…
Saturday November 15, 2025