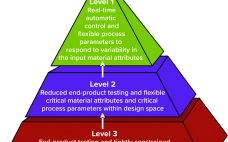
Process control enables biomanufacturers to ensure that operating parameters are within defined specifications. A control strategy should be established during early stages of process development while process and product performance are being defined using risk-based methods such as quality by design (QbD) and process analytical technologies (PATs). Confirming process control as an essential part of product development creates greater process knowledge and understanding and provides the first steps toward process optimization. By understanding how process performance relates to product quality,…
Author Archives: Peter Satzer
Making Downstream Processing Continuous and Robust: A Virtual Roundtable
Current biomanufacturing is driven to pursue continuous processing for cost reduction and increased productivity, especially for monoclonal antibody (MAb) production and manufacturing. Although many technologies are now available and have been implemented in biodevelopment, implementation for large-scale production is still in its infancy. In a lively roundtable discussion at the BPI West conference in Santa Clara, CA (11 March 2019), participants touched on a number of important issues still to be resolved and technologies that are still in need of…