Since 2001, global contract development and manufacturing organization (CDMO) CMC Biologics has completed more than 120 projects with at least 100 pharmaceutical partners. During that time, the company has taken a holistic approach to helping clients balance manufacturing risks and rewards. The team focuses on evaluating key technologies to deploy a constantly evolving set of capabilities in support of biopharmaceutical clients throughout their product lifecycles. Part of that commitment is continually evaluating what would best benefit customers and where key…
Author Archives: Peter Levison
Significant Technology Advances Enabling Integrated Continuous Bioprocessing
Peter Levison (senior director, Pall Life Sciences ) BPI Theater @ INTERPHEX April 26, 2016, 1:40–2:00 pm Pall’s advancements in integrated continuous bioprocessing include new product launches. The company has built a continuous laboratory at its facility in Westborough, MA. It is 6 m × 6 m and can produce >2 g of pure monoclonal antibody (MAb) per hour. One continuous process flows through one step to the next, from cell culture to formulation. Continuous manufacturing is being used successfully…
Integrated and Scalable Continuous Chromatography: The Cadence BioSMB PD System from Pall
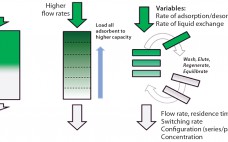
Bioprocess intensification leads to more efficient, flexible, and cost-effective biopharmaceutical manufacturing facilities. Single-use systems designed for continuous operation facilitate process intensification. Pall Life Science’s Cadenceâ„¢ BioSMB PD platform is the first disposable flow path, continuous multicolumn chromatography solution that is fully scalable from a process development laboratory to good manufacturing practice (GMP) manufacturing. It provides an economically viable, disposable chromatographic solution for even the most demanding and costly chromatographic steps. During multicolumn countercurrent (also known as simulated moving bed, or…
Moving One Unit Operation At a Time Toward Continuous Biomanufacturing: An Overview of Pall Solutions for Integrated Continuous Biopharmaceutical Production
Numerous industries have demonstrated that continuous manufacturing provides significant benefits and advantages, ranging from reduced capital and operating expenses to greater efficiency and product quality and consistency. The conventional approach to biopharmaceutical manufacturing involves the batch performance of a series of unit operations separated by hold steps requiring additional tanks and/or biocontainers. Such an approach fails to maximize facility use, requires large buffer volumes, and results in overall inefficiencies. An integrated, continuous approach to bioprocessing connects each unit operation, minimizing…
Continuous Chromatography Is Now Possible for Clinical Manufacturing
Intensified and integrated bioprocess technologies are creating a paradigm shift toward more efficient, higher flexibility facilities for biopharmaceutical manufacturing. Continuous technologies that are designed as single-use systems help to greatly facilitate process intensification, delivering further efficiencies with reduced set-up times and elimination of the need for cleaning and cleaning validation. Chromatography is often considered to be a challenging bioprocess step, which has caused great interest in a simplified, safer solution. Continuous multicolumn chromatography using a single-use flow path is an…
Development of a High-Performance, Integrated, and Disposable Clarification Solution for Continuous Bioprocessing
Current bioprocesses combine fed-batch cell culture with batch-wise downstream processing steps. To achieve integrated upstream and downstream continuous manufacturing, the industry has been in need of a continuous cell separation and clarification solution for bioprocess fluids from bioreactors. The Cadence Acoustic Separator from Pall Life Sciences provides this solution, with continuous first-stage clarification without the need for filter media in a scalable, single-use format with no negative impact on product attributes. The Cadence Acoustic Separator delivers cost and time savings…