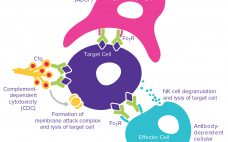
The complexities of biomanufacturing combined with heterogeneity introduced by cellular expression systems present significant challenges to assessing the quality of biologics such as monoclonal antibodies (MAbs). Information related to the critical quality attributes (CQAs) of MAb drug candidates is unknown during early phase drug development. It must be established empirically by physical, structural, and functional analyses as early as possible to accelerate development and mitigate risk through greater understanding of product characteristics. High-resolution analytical techniques are required to answer questions…
Sunday April 06, 2025