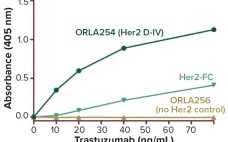
To meet the ongoing need for new and improved drugs, the biopharmaceutical community strives to create molecules with new functions. Bispecific antibodies (bsAbs), which can simultaneously home in on two different targets, illustrate the scientific ingenuity needed for this task. The basic proof of concept for these complex molecules was established in 1960 (1), and their application to the redirection of effector cells was reported in the mid-1980s (2–4), but producing them has proved to be challenging. Many technical advances,…
Author Archives: Nick Hutchinson
eBook: Bispecific Antibodies — Their Development and Manufacture As Therapeutics
Generating antibodies with two or more specificities is one of the most innovative fields in therapeutic antibody development, with tremendous potential for use in creating new treatments for patients with unmet medical needs. In particular, bispecific antibody development is stimulating innovations in bioprocessing techniques from expression through upstream processing and candidate purification. Wherever possible, process-development scientists and engineers are borrowing techniques that were honed for mature monoclonal antibody (MAb) platforms, then applying those to bispecific antibody manufacturing. Nevertheless, the unique…
Tracking Therapeutic Antibody Development in a Pandemic
The COVID-19 pandemic has generated a significant and rapid response from scientists who aim to develop drugs and vaccines in the academic, government, and industrial sectors. Such interventions are essential to containing SARS-CoV-2, the coronavirus that causes the COVID-19 disease. To inform and educate the public about global efforts to develop targeted therapies such as monoclonal antibodies (MAbs), The Antibody Society (TAS) and the Chinese Antibody Society (CAS) have designed and implemented a freely available online database called the COVID-19…
Analytical Tools to Improve Decision-Making During Product Development
Speed to clinic testing — and then speed to market — are highly significant metrics for companies developing biopharmaceuticals. By increasing the pace of drug development, these companies can reduce costs, obtain revenues early, and establish commanding positions in the market relative to their competitors. High-throughput development tools have contributed much to the acceleration of drug development in recent years. Such technologies enable the testing of many process parameters in parallel. Combining them with multifactorial “design of experiment” (DoE) analysis…
Final Thoughts: Single-Use Platforms
The development and launch of new biopharmaceutical products is a very challenging and risky process. Sponsors must get their products into clinical testing as quickly as possible to beat their competition and take the greatest share of the market. Yet that need for speed must not lead to companies launching products with inefficient manufacturing processes that ultimately will leave them vulnerable to attack from low-cost competition. Single-use systems have been a significant enabling technology for companies developing new biologics because…
Continuous Cell Culture Operation at 2,000-L Scale
In the biopharmaceutical industry, continuous manufacturing is often cited as a method for increasing the productivity of bioprocesses (1). Compared with batch processing, it has the potential to enable production of more product within a smaller facility footprint — while improving product quality, particularly for sensitive and unstable molecules. Investigation into continuous methods is taking place for both upstream and downstream operations. For the full benefit of continuous processing to be realized, an argument has been made that cell culture,…
Single-Use Tangential-Flow Filtration Can Save Five Hours of Facility Time
Single-use technologies can be applied to tangential-flow filtration (TFF) applications within bioprocessing. In such applications, they can reduce the need for utilities, reduce capital expenditure during early phases of drug development, increase processing flexibility during process design and operation, and limit product cross-contamination risks. Read the full text of this application note in the PDF (Login required).
Automation of a Single-Use Final Bulk Filtration Step: Enhancing Operational Flexibility and Facilitating Compliant, Right–First-Time Manufacturing
Single-use technologies have been implemented in biomanufacturing facilities all over the world. Inherently more flexible than stainless steel equipment, single-use technology allows for more rapid technology transfer by minimizing the time it takes to design, purchase, and qualify new capital assets. Rapid turnover between batches is facilitated with no need for protracted clean-in-place (CIP) and steam-in-place (SIP) regimes; the risk of product cross contaminations is reduced because single-use fluid-contact surfaces are never previously exposed to a biopharmaceutical product stream. For…
Successful Implementation of Automation in Single-Use Bioprocessing
Single-use bioprocessing is now common for the production of biopharmaceuticals. Existing and future biopharmaceuticals are extremely complex molecules that display structural heterogeneity. This heterogeneity must be carefully controlled in order to deliver consistently safe and efficacious product to patients. The FDA’s Guidance for Industry on Process Validation states that the manufacturer should understand the sources of variation be able to detect variation and understand the impact of variation on the process and ultimately the product. This presentation will review sources…
Understanding and Controlling Sources of Process Variation: Risks to Achieving Product Critical Quality Attributes
Biopharmaceuticals include recombinant proteins, vaccines, gene therapies, and drug products derived from stem cell technology. One key characteristic of shared by all biologics is that they tend to be extremely large molecules with complex three-dimensional structures, critical to their functionality. For example, monoclonal antibodies (MAbs) are composed of more than one thousand times larger than a molecule of aspirin (one analogy compares the complexity of a MAb to that of an F16 jet, and the complexity of aspirin to that…