Monitoring critical process parameters (CPPs) and key performance indicators in bioreactor control systems is crucial to ensure proper cell growth and protein production. Today, most of the major biopharmaceutical companies employ capacitance measurement, in R&D and through process development to manufacturing. Owing to the increased use of single-use bioreactors and building on Aber’s experience with single-use capacitance sensors, the latest Futura neotf single-use capacitance sensors have been specifically developed for integration into Thermo Fisher Scientific bioprocess containers (BPCs) for use…
Author Archives: Nephi Jones
Measuring Cell Density in HyPerforma S.U.B.s with ABER Futura neotf
Single-Use Bioreactors: Performance and Usability Considerations, Part 2
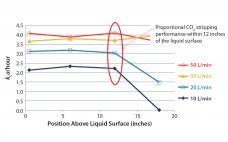
As the biopharmaceutical industry continues toward streamlined bioprocessing and intensified cell-culture biology, selection criteria of single-use bioreactors (S.U.B.s) and other bioprocessing technologies will become increasingly rigorous, emphasizing the importance of considering every aspect of technologies under evaluation. In part 1, we discussed performance for process control, including the maintenance of critical process parameters (CPPs), and highlighted bioreactor performance (e.g., mass transfer, power per volume, and temperature control) as a critical consideration during the selection of S.U.B.s (1). Part 2 focuses…
Single-Use Bioreactors: Performance and Usability Considerations Part 1: Performance for Process Control
There is ever increasing pressure for the biopharmaceutical industry to drive toward higher efficiency and lower costs. Compared to the past, target markets for many drugs typically are becoming smaller, and so-called blockbuster drugs are becoming more the exception than the rule. Regulatory agencies have continued to increase the pressure on drug makers to meet increasing quality standards and accept higher levels of responsibility. Furthermore, customer pricing, healthcare markets, and recent biopharmaceutical pricing scandals all add incentives toward more efficient…
Simplify Upstream Process Development and Scale-Up: Single-Use 5:1 Turndown-Ratio Bioreactor Technology
Single-use technologies (SUTs) have been adopted widely in the biopharmaceutical industry for product development as well as clinical- and commercial-scale manufacturing. Over the years, suppliers of such equipment have addressed concerns about waste management, extractables and leachables, and reliability of supply — and as a result, end users have gained confidence in SUTs. Recognizing potential benefits that can be realized for both clinical and commercial operations, biomanufacturers increasingly are implementing SU solutions at larger scales in both upstream production and…
High Density Culture Strategies For Improved Scalability With Single-Use Systems
Improvements in single-use systems have allowed implementing high-density cultures in standard work flows. The current study shows integration of the Thermo Scientific™ HyPerforma™ Single-Use Bioreactor (S.U.B.) and the XCell™ ATF6 Single-Use (SU) System to achieve high-density cultures. Current results are compared against similar cultures using a stainless steel ATF6 system. The S.U.B. was able to support high-density cultures (>40E06 cells/mL) without modification to standard single-use components and maintained proper operating parameters. Scale-up criteria for both S.U.B. and ATF are provided…
Single-Use Processing for Microbial Fermentations
During the past decade, single‑use bioprocessing has emerged as a standard platform for current good manufacturing practice (CGMP) mammalian cell culture. Biomanufacturers have come to appreciate the benefits of lower capital and operating costs, reduced contamination risk, continuity from early development through manufacturing, flexibility, and sustainability (1). Disposable cell‑culture vessels have gained wide acceptance because their performance duplicates that of stainless‑steel, fixed‑tank bioreactors, with which manufacturers have extensive experience. This is no accident: Single‑use bioreactors use stainless–steel engineering principles, particularly…