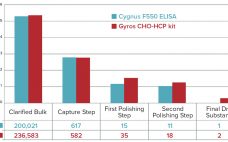
In the past few years, increasing numbers of biotherapeutics have been approved for market (1). Among all the regulatory concerns for commercial biotherapeutics, host-cell proteins (HCPs) are a major class of process-related impurities that remains a critical quality attribute (CQA) for bioprocess development because of associated risks to product quality, safety, and efficacy. HCP identification, clearance, assay setup, and process control are critical points for health authorities, and many guidelines aim for better control of HCP content in final biologic…
Saturday November 15, 2025