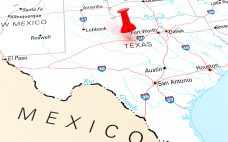
CDMO VGXI will increase plasmid DNA capacity by over 500% through an investment at a site in Conroe, Texas. The contract development manufacturing organization (CDMO) did not divulge any financial details associated with its new headquarters and manufacturing plant. However, the facility will be located at Deison Technology Park in Conroe, Texas on a 21.5-acre area of land. “The new facility is purpose built to support VGXI’s optimized Plasmid DNA production process at expanded scales and across multiple flexible manufacturing…
Author Archives: Millie Nelson
Moderna trialing refrigerator stable COVID-19 vaccine
Moderna has another COVID-19 vaccine in its clinical pipeline, which if successful will potentially make distribution and administration easier. The firm already has an mRNA cell-free COVID-19 vaccine, 1273, approved under FDA emergency authorization. However, it is developing an additional COVID-19 vaccine candidate coded 1283, which it believes will be easier to allocate and administrate due to it potentially being refrigerator stable. According to the firm, the next generation COVID-19 vaccine candidate is made from portions of the spike protein,…
Ins & outs: High level leadership changes at Novavax
Novavax has switched up its leadership with multiple changes. Meanwhile there are changes at Kyverna and Precision BioSciences. Put your feet up and enjoy BioProcess Insider’s Ins & Outs. US-based biotechnology company Novavax develops and commercializes various vaccines to treat infectious diseases such as Ebola, seasonal influenza, and COVID-19. The firm has its own COVID-19 vaccine candidate, NVX-CoV2373, which has not yet received approval from the UK’s Medicines and Healthcare products Regulatory Agency (MHRA) and is currently in two Phase III…
Catalent adds cryogenic capabilities at Philadelphia facility
CDMO Catalent has invested to expand cryogenic capabilities at its clinical supply services facility in Philadelphia to meet cell and gene therapy demand. The contract development manufacturing organization (CDMO) did not disclose any financial details of the investment. However, a spokesperson for Catalent told BioProcess Insider “In addition to the equipment necessary to handle, package and label cryogenic materials, Catalent will add five cryogenic freezers with enough capacity for tens of thousands of vials at very low temperatures of around…
CDMO round-up: News from Lonza, MedPacto and Revolo Biotherapeutics
Lonza collaborates with Immunitas to develop candidate; MedPacto signs agreement with Samsung Biologics; Revolo Biotherapeutics chooses CordenPharma to manufacture its peptide and immune system resetting drug candidate. Great to have you here for Bioprocess Insider’s CDMO round-up. First up in our contract development manufacturing organization (CDMO) round-up is Lonza, which recently entered an agreement with Immunitas Therapeutics to support manufacturing of Immunitas’ lead program, IMT-009. IMT-099 is a monoclonal antibody that suppresses NK cells and T cells in the tumor microenvironment and…
Albumedix more than doubles albumin production with expanded UK plant
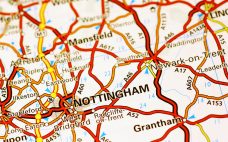
Albumedix has completed expansion of its commercial-scale manufacturing facility and established new labs, doubling its albumin production capacity. After more than three years of design and construction work, Albumedix has completed the expansion of its Nottingham facility and is now able to manufacture out of this site. The expansion enables the firm to more than double its albumin production, which is used for stabilization and to deliver pharmaceuticals. “Part of the expansion included the construction of a four-storey building adjacent…
Abzena selects NC for $200m biologics plant
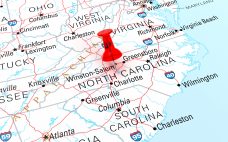
Abzena has selected Sanford, North Carolina as the site of a biologics manufacturing facility equipped with 12x 2,000 L bioreactors. In January, British contract development manufacturing organization (CDMO) Abzena announced the addition of a sixth global site in the US. The exact location of the plant was not divulged. However, the firm revealed this week that the site will be in Sanford, North Carolina. The Sanford facility will cost over $200 million and will be dedicated to manufacturing mammalian biologics.…
Catalent completes Wisconsin expansion and doubles its capacity
CDMO Catalent has completed an $85 million expansion at its Madison drug substance facility and has started work on customer programs. The contract development manufacturing organization (CDMO) Catalent has added two mammalian cell culture suites at its Madison facility, meaning the site has five suites in total and has more than doubled its overall capacity. The two additional suites each boast a 2 x 2,000 L single-use bioreactor system that can process batches of 2,000 L or 4,000 L for…
Ins & outs: Cavazzoni named as director of FDA’s CDER
The US FDA’s CDER  has named Patrizia Cavazzoni as its permanent director. Meanwhile, there are high level changes at Rentschler Biopharma, Bone Therapeutics, and Imvax. Sit back, relax, and enjoy BioProcess Insider’s Ins & Outs. The appointment comes after Cavazzoni has served as acting director of the US Food and Drug Administration’s Center for Drug Evaluation and Research (FDA’s CDER) for the past year after Janet Woodcock stepped away from the role to assist Operation Warp Speed (OWS) in response to…
Lonza to make Junshi Biosciences antibody at China facility
Lonza has expanded its collaboration with Junshi Biosciences to develop and manufacture an antibody candidate at its recently opened, Guangzhou facility. Swiss contract development manufacturing organization (CDMO) Lonza has extended its partnership with Chinese firm Junshi Biosciences to advance and manufacture biologics. The agreement also includes the production of an antibody candidate that will be manufactured at Lonza’s site in Guangzhou, China. Under the agreement, Lonza will provide support to develop and manufacture current and future antibody-based products in Junshi…