The partnership with Aldevron has led to an mRNA breakthrough with their manufacturing approach proving 10 times more efficient than the previous process, says Ginkgo Bioworks. The collaboration, which was formed earlier in 2021, has resulted in what the firms have called a breakthrough for Vaccinia Capping Enzymes (VCEs) used to manufacture messenger RNA (mRNA) vaccines. VCEs cause the human body to recognize the RNA as something it can translate into a protein, which is essentially how the vaccine works.…
Author Archives: Millie Nelson
Artiva to build Californian NK cell therapy manufacturing facility
The facility in San Diego will support off-the-shelf NK and CAR-NK therapeutic manufacturing to treat solid and haematological cancers, the firm says. Construction of the 52,000 square-foot facility is already underway and is expected to open in 2022. The plant adds to Artivaâs cell therapy manufacturing site in South Korea. COO of Artiva Peter Flynn told BioProcess Insider that the site in Korea will continue its operations and the San Diego facility âis an additional site, it is not a…
MaxCyte to support Sanaâs ex vivo cell therapy plans
Sana will use MaxCyteâs Flow Electroporation technology and ExPERT platform to drive its hypo-immune cell therapy pipeline. The agreement, of which financial details have not been disclosed, sees MaxCyte sign a clinical and commercial license with Sana Biotechnology. Sana will gain non-exclusive commercial and clinical rights to use MaxCyteâs Flow Electroporation technology and ExPERT platform and in return, MaxCyte is eligible to receive licensing fees and milestone payments. âMaxCyteâs ExPERT platform is based on the companyâs proprietary Flow Electroporation,â MaxCyte…
BeiGene sets sights on US with NJ manufacturing facility
BeiGene plans to build an R&D and biomanufacturing facility at a 42-acre site in Hopewell, New Jersey. The deal, of which financial details have not been disclosed, sees BeiGene enter into a purchase agreement to acquire a site at the Princeton West Innovation Campus where it will construct a commercial-stage biologic manufacturing and clinical R&D facility. Hopewellâs central location and closeness to a pharmaceutical research, development, and manufacturing talent pool were cited by the firm as reasons to expand its…
Appia partners with Kite to develop cell therapies for blood and bone cancers
Kite will develop CAR-iNKT cells  for the treatment of haematological malignancies using Appia Bioâs ACUA technology platform. The deal, which could be worth up to $875 million, will see Appia responsible for preclinical research of two hematopoietic stem cell (HSC) derived chimeric antigen receptor-engineered invariant natural killer T (CAR-iNKT) product candidates manufactured with CARs provided by Kite. âThe core of the partnership is that we will bring together Appiaâs cells and Kiteâs CARs, putting us right at innovationâs edge.  We…
MilliporeSigma: ‘Viral vectors will dominate the next decadeâ
Conversations like this usually happen on the floor of exhibition centers. But with face-to-face events remaining in limbo, BioProcess Insider spoke virtually with MilliporeSigmaâs head of cell and gene therapies Jerry Keybl about the future of viral vectors. Â Keybl has been looking after the business for over four years and is focused on driving industrialization to make sure that MilliporeSigma has the solution from a product and service prospective. Bioprocess Insider (BI): What are the complexities of manufacturing viral…
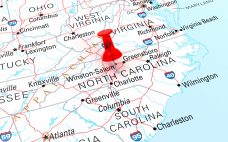
Amgen forks out $550m to build drug substance facility in NC
The facility in Holly Springs, North Carolina will support stainless steel-fed batch manufacturing and single-use technologies making the plant more flexible, the firm says. The $550 million investment comes one week after Amgen announced plans to construct a $365 million plant in New Albany, Ohio to support drug packaging. âWe are building the facility to support the growing demand for Amgen medicines in the US market,â an Amgen spokesperson told BioProcess Insider. âThis new âFleXBatchâ drug substance plant will increase…
Bayer buys Vividion for $1.5bn in âarmâs length acquisitionâ
Bayer bolsters its drug discovery capabilities through the acquisition of Vividion, which it says will benefit from an armâs length business model. The deal â which could be worth up to $2 billion – strengthens Bayerâs small molecule oncology and immunology targets through the addition of Vividion Therapeuticsâ chemoproteomic technology. According to German pharma giant Bayer, the platform can recognize unidentified binding pockets on protein targets by screening chemical probes against the human proteome to establish selectivity. The platform is…
Sanofi bolsters mRNA capabilities with Translate buy
Sanofi will acquire its partner Translate Bio for $38.00 per share, as it looks to drive mRNA technology for therapeutics and vaccines. Under the terms of the deal, Sanofi will acquire Translate for a total equity value of approximately $3.2 billion as the French pharma giant builds on its plans to invest â¬400 million ($475 million) annually into what it describes as its âmRNA Center of Excellenceâ to accelerate R&D of next-generation vaccines. In 2018, Sanofi teamed up with Translate…
Ins & Outs: âCDMOs are now the leaders in the biomanufacturing spaceâ
BioProcess Insider figuratively sat down with bioprocess veteran Roger Lias, as the new chief commercial officer of Vibalogics, to discuss his different experiences and predictions for the CDMO market. Lias has served in senior roles at Diosynth, Lonza, KBI Biopharma, Cytovance Biologics and Eden Biologics. More recently Lias served as chief executive officer at Avid Bioservices before joining Indian drugmaker Stelis Biopharma, where he was responsible for transitioning its manufacturing models. His latest career move takes him to US and…