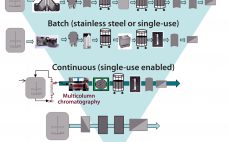
The BioPhorum first-edition Technology Roadmap outlined a 10-year vision for therapeutic protein production in the biopharmaceutical industry (1). The roadmap describes multiple manufacturing scenarios ranging from large-scale (~20,000-L production) to small and agile, portable production facilities. It includes detailed analyses of the needs for the future in each of the following areas: Process technologies (2) Inline monitoring and real-time release (3) Automated facilities (4) Modular and mobile (5) Knowledge management (6) Supply partnership management (7). Since the 2017 publication of…
Author Archives: Mark Brower
Standardized Economic Cost Modeling for Next-Generation MAb Production
Historically, in generating material for clinical testing during antibody process development, emphasis was placed on efficacy, product quality, regulatory compliance, and speed. As the biopharmaceutical industry has matured (and with increasing competition), emphasis has shifted toward cost optimization and manufacturability. Reducing the costs of medicines for patients and payers (thereby broadening access to drugs) is now a key driver during development of new therapies as well as modernizing processes for existing molecules. Cost reduction includes providing robust manufacturing processes that…