Recent advances in molecular biology are expediting genomic sequencing to underpin precision medicine. Such progress is positioning gene and gene-modified cell therapy on the cusp of an extraordinary revolution in patient care for presently unmet medical needs — and a new therapeutic class that could rival monoclonal antibodies (MAbs) in importance. However, despite substantial strides made in clinical trials, the bioprocessing community is struggling to fulfill growing demands for biomanufacturing capacity to make gene and gene-modified cell therapies — including…
Author Archives: Kim Bure
Automation of CAR-T Cell Adoptive Immunotherapy Bioprocessing: Technology Opportunities to Debottleneck Manufacturing
Continued clinical efficacy demonstrations of cell-based immunotherapies (iTx) such as chimeric antigen receptor T cell (CAR-T) therapies has made the prospect increasingly likely of an immunotherapy product achieving conditional market authorization in the short term. For example, Novartis and the University of Pennsylvania’s lead candidate (CTL019) for treating a range of hematological malignancies received breakthrough status from the US Food and Drug Administration (FDA) in 2014, permitting access to an expedited drug development pathway for high unmet medical needs (1).…
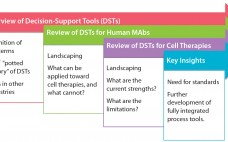
Decision-Support Tools for Monoclonal Antibody and Cell Therapy Bioprocessing: Current Landscape and Development Opportunities
Industrial-scale manufacturers in a number of fields — from automobiles to biotherapeutics — have long relied on powerful computational and mathematical tools to aid in the scale-up, optimization, quality control, and monitoring of product development (1–5). Typical process pathways are highly multifactorial, with numerous branch points, feedback steps, instrumental attributes, and target parameters. Moreover, margins for error are minimal for most industrial processes, requiring high standards of precision from industrial and operational pathways (6). For those reasons, the complexity of…
Quantitative Risk Assessment of Bioaccumulation Attributable to Extractables and Leachables in Cellular Immunotherapy Biomanufacturing
Precious patient samples, contamination concerns, and limited product purification options have compelled manufacturers of cellular immunotherapies (iTx) such as chimeric antigen receptor T cells (CAR-T) and T-cell receptor (TCR) technologies toward the disposables industry. Such companies are implementing single-use technologies (SUTs) almost exclusively (1). But despite the dominance of disposable bioprocess platforms and their extraordinary growth in the iTx marketplace, researchers have made limited efforts to understand the perennial and critical bioprocessing risks of leachables and extractables. Here we outline…