Advanced therapy medicinal products (ATMPs) are engineered to replace defective, disease-causing genes to compensate directly for a genetic defect or to encode a therapeutic protein construct (e.g., chimeric antigen receptor, CAR) for disease treatment. In most instances, a viral vector delivers the engineered genetic payload, targeting cells in situ or ex vivo through cellular modification, expansion, and infusion into a patient. Clinical successes of ATMPs bolstered by regulatory approval of products such as Luxturna (voretigene neparvovec-rzyl, Spark Therapeutics), Kymriah (tisagenlecleucel,…
Author Archives: Joshua Speidel
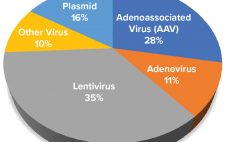
AAV Vector Manufacturing Platform Selection and Product Development
Adenoassociated virus (AAV) vectors have emerged as the prominent delivery mechanisms of corrective gene therapies. Three such products — Glybera (alipogene tiparvovec, uniQure), Luxturna (voretigene neparvovec-rzyl, Spark Therapeutics), and Zolgensma (onasemnogene abeparvovec-xioi, AveXis) — have been licensed, and a growing number of candidates are entering late-stage development. In mapping out an AAV gene therapy product development strategy, biomanufacturers should address fundamental considerations for their manufacturing strategies for both phase 1–2 clinical evaluation and translation for commercial market supply. A manufacturing…
Seamless Transition from R&D to Manufacturing
Fast and cheap: These criteria are becoming ever more urgent drivers for manufacturers of biologics, faced with increased scrutiny on the costs of developing novel drugs, the lengthy timelines for delivering these drugs to patients, and the tightening competition to capitalize on new targets. The challenge for manufacturers is further heightened by the expectations to deliver on quality as well. Although development and production of molecules such as monoclonal antibodies (MAbs) have greatly benefited from the “platformization” of core technologies…
On-Demand Product Development Teams
A BPI Theater Roundtable at the 2016 BIO Convention On Wednesday, 8 June 2016, Joshua Speidel (managing director of the commercial practice team at the Latham Biopharm Group) chaired an afternoon roundtable titled, “On- Demand Product Development Teams.” He brought together a panel of four experts: Matt Rebold (director of business development at ICON Imaging and Technology — West Coast) Paul Jojorian (global head of technology transfer for Patheon Biologics) Eiry Wyn Roberts (vice president of research and development for…