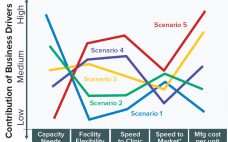
Drug Substance Scenarios Given the complexity of the biopharmaceutical industry and the increasing diversity of products and companies, it is clear that there will be no “one size fits all” solution to biomanufacturing. Instead, we see a range of biomanufacturing scenarios playing out over the next 10 years. Five high-level scenarios were selected for drug substance manufacturing and two for drug product to cover the full spectrum of process and facility types. Each facility type is associated with a representative…
Author Archives: Jonathan Souquet
Seamless Transition from R&D to Manufacturing
Fast and cheap: These criteria are becoming ever more urgent drivers for manufacturers of biologics, faced with increased scrutiny on the costs of developing novel drugs, the lengthy timelines for delivering these drugs to patients, and the tightening competition to capitalize on new targets. The challenge for manufacturers is further heightened by the expectations to deliver on quality as well. Although development and production of molecules such as monoclonal antibodies (MAbs) have greatly benefited from the “platformization” of core technologies…