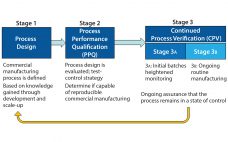
As defined in the ICH Q10 guideline, a control strategy is “a planned set of controls, derived from current product and process understanding, that assures process performance and product quality” (1). Every biopharmaceutical manufacturing process has an associated control strategy. FDA’s 2011 guidance for process validation (2) describes process validation activities in three stages (Figure 1). A primary goal of stage 1 is to establish a strategy for process control that ensures a commercial process consistently produces acceptable quality products.…
Sunday October 05, 2025