Process intensification offers many benefits but implementing PI can introduce an unexpected challenge of managing larger media volumes. This whitepaper helps ensure your implementation strategy accounts for the increased media volume associated with process intensification. What You Will Learn Gain the tools and knowledge needed to confidently assess intensification options with a focus on media management for new or existing facilities. Follow the media journey from prep to use, exploring potential logistical pitfalls in the management of increased media volumes…
Author Archives: BPI Contributor
Practical Approaches to Metals Analysis of Cell Culture Media & Impact on Therapeutic Protein Production Using ICP-MS
This webcast features: Dr. Adil Mohammad, Staff Fellow, US FDA, Dr. Chikkathur Madhavarao, Biologist, US FDA, Robert Thomas, CSci, CChem, FRSC, Principal Consultant, Scientific Solutions Biotechnology products (biologics) are often produced from mammalian cells grown in large-scale bioreactors. The dynamic environment within the bioreactor is comprised of growth media along with cells, proteins, nutrients of organic and inorganic origin, metabolic waste products and metal ions. Metal ions can act as enzyme cofactors and can directly influence the kinetics of biochemical…
A Frost & Sullivan Virtual Think Tank: 3M™ Polisher ST Beta Testing Series
One step can change everything: Replace your flow-through AEX column with a novel single-use polishing solution 3M™ Polisher ST single use polishing solution – from lab to manufacturing scale Frost & Sullivan recently invited a panel of bioprocessing industry leaders and key opinion leaders to participate in a Virtual Think Tank (VTT) Early Access series – a new and unique thought leadership forum. Each VTT panel, comprised of professionals from top pharmaceutical companies, contract development and manufacturing organizations (CDMOs) and…
Build vs. Buy: A Critical Calculation for Cell Therapy Innovators
Cell therapy is proving to be one of the most promising advanced modalities, representing a significant step forward in the treatment of a wide range of challenging diseases and conditions. As a cell therapy candidate advances from discovery through clinical development and ultimately to commercialization, foundational decisions must be made by the product sponsor that will impact both scientific and commercial success. One of the biggest decisions is whether to build a manufacturing facility or outsource to a contract development…
Adenovirus Vector Manufacturing Platform Using CIMmultus QA
This webcast features: Hana Jug, Project Manager in Process Development for Viral Vectors and Vaccines, BIA Separations Downstream processing remains one of the main bottlenecks in Adenoviral vector manufacturing. At BIA Separations, a Sartorius company, we offer a platform for purification of Adenoviral vaccines using market leading CIM® monolithic chromatographic columns and an analytical toolbox for process monitoring in Adenoviral vaccine production. Simplified purification of Adenovirus consists of typical downstream steps, including combined lysis, clarification, TFF and a chromatographic capture…
Five Ways the Amersham ImageQuant™ 800 GxP Helps You Remain Compliant
In highly regulated environments, such as the pharmaceutical industry, remaining GxP compliant while efficiently delivering your products to a competitive market is a challenging task, particularly when Western blot imaging is involved. While this can often be a tricky and time-consuming task, our Amersham ImageQuant™ 800 GxP imaging system and software streamlines the process. It provides simple solutions that support data traceability, accountability, and integrity. Confident decision making and GxP compliance go hand in hand with high-quality imaging. In this…
Optimization of an Anion Exchange Method for the Large-Scale Production of Plasmid DNA for Gene Therapy and DNA Vaccine Applications
This webcast features: Jenny England, Staff Scientist, Innovation & Applications, Thermo Fisher The advancement of gene therapy and plasmid DNA (pDNA) vaccines has highlighted the need for the development of a large-scale purification process to produce high purity plasmid DNA. Plasmid DNA serves as a foundation to produce viral vectors such as adenoassociated virus (AAV) or lentivirus (LV), a template for mRNA production during the in vitro transcription reaction and can be used directly as a pDNA vaccine. The typical…
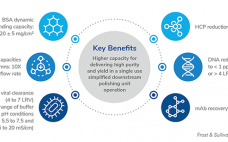
3M™ Polisher ST — The Next Frontier in Downstream Processing, a Frost & Sullivan White Paper
Advancements in next-generation therapies—Paving the way for novel bioprocessing solutions. Learn about 3M Polisher ST technology which utilizes a guanidinium-functionalized polyamide membrane protected by a Q functionalized non-woven material. This Frost & Sullivan whitepaper explains how the technology demonstrates the viability of replacing the depth filtration and anion exchange chromatography (AEX) steps to achieve a simplified and cost-effective process. The platform is designed to reduce process and product related impurities and offer robust performance across a wide range of process…
Analytical Ultracentrifugation for Profiling AAV Gene Therapies: Where Are We Now?
This webcast features: Yijun Huang, Scientific Fellow, WuXi Advanced Therapies Adenoassociated virus (AAV) is often the vector of choice for gene therapies due to their low immunogenicity, ability to promote long-term gene expression, and capsid tropism-dependent tissue specificity. Representing around 37% of the current advanced therapies market, there is an ongoing demand for robust quality lot release programs capable of supporting the large patient cohorts involved in clinical trials. Along with critical quality attributes such as titer, identity, and potency,…
Scale-Down Optimization to Scale Up Success
As immune cell therapy advances to address new indications, the need for rapid development of robust manufacturing processes becomes increasingly important. Early process optimization sets the stage for clinical and commercial manufacturing and plays a foundational role in decreasing time to market and lowering COGS. This application note describes the streamlining of T-cell expansion optimization using a DOE-based approach in a semi-automated, controlled multi-parallel setup of the Sartorius T-Cell Exploration and Characterization Solution. Download to learn about: Rapidly screening media…