Sekisui XenoTech is a global contract research organization based in Kansas City, KS. The company hired a record number of new staff over the past year in order to meet current demand for its products and services, which are utilized to optimize the discovery, development, and approval of new drugs and other compounds. Several previous Sekisui XenoTech employees also returned to the company. The new staff span Sekisui XenoTech’s contract research, quality assurance, business, IT, and other departments. “Our hiring…
Author Archives: BPI Contributor
We Meet Your Single Use Process Monitoring Requirements
Innovation is at the core of all pharmaceutical, biopharm and bioprocess applications. With extensive experience in the biopharmaceutical industry — and particular expertise in single-use bio-pharmaceutical equipment — PendoTECH delivers a line of pressure sensors, control systems and software for measuring, monitoring and data collection in bioprocess applications and other areas where the products provide a unique process solution. Committed to meeting the specialized needs of life-science laboratories and bio-pharmaceutical manufacturers, PendoTECH technical consultants review your company’s applications in the…
JSR Announces Acquisition of Crown Biosciences International
JSR Life Sciences (JLS) announced today that JSR Corporation has agreed to purchase Crown Bioscience International (TPEx: ticker 6554) at 12 Billion New Taiwan Dollar, a global drug discovery and development services company providing translational platforms to advanced oncology, inflammation, cardiovascular and metabolic disease research. This acquisition marks JSR’s largest life sciences-focused investment to date, extending the company’s portfolio to include industry-leading contract research and development capabilities, and ability to develop impactful solutions for pharma. As a premier contract research…
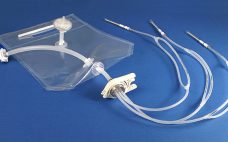
AdvantaPure® Introduces Single-Use Filling Assemblies For Aseptic Fill & Finish Applications
New sterile molded filling assemblies from AdvantaPure continue the shift to single-use processing methods that save time, reduce cross contamination risks and increase productivity between batches of biopharmaceutical and pharmaceutical products. “These assemblies were developed specifically for vial and syringe filling,” notes Lawrence Morano, AdvantaPure’s global sales manager. “Our team of engineers is well versed in single-use, biopharmaceutical manufacturing, and fluid flow, and we’re able to work closely with customers to design systems that meet their individual process requirements.” Manufactured…
U.S. Approval of Three Rapid Microbiological Methods for MACI Product Release
Short time frames are a major challenge in developing alternative microbiological methods for autologous cell therapy products. Ideally, results are made available in under a day. Obtaining regulatory acceptance also can be a challenge, but it is made easier if methods are included in an application (e.g., a biologics license application, BLA) rather than changing a method that is already part of an approved process. Comparing different detection platforms can be a challenge if they have different readouts, and validation…
Comparing Adherent-Cell Technologies: Amplification of Virus Stocks and Viral Vectors
FUJIFILM Diosynth Biotechnology (FDB) is a multifunctional commercial development manufacturing organization (CDMO) that manufactures virus-based therapeutics. Its site in Texas specializes in both adherent and suspension cell culture for production of virus vectors and for development of analytical assays to support production and expression of our clients’ therapeutics. We work with a number of cell lines such as human embryonic kidney (HEK293) and human retinal cells (Johnson & Johnson/Crucell’s proprietary PER.C6 line), which can grow in suspension. However, many of…
GEA Optimizes Monitoring of Powder Flow, Fluidization and Cleaning
GEA Service recently launched its new VISIOCOVER™ Upgrade Program for the safe visual inspection of non-sticky-powder processes in the dairy, food, chemical and pharma industries. For customers currently using SANICOVER™ to monitor their powder flow, fluidization and cleaning, the upgrade provides three standard sizes to match the SANICOVER™ systems. Installing the VISIOCOVER™ on existing SANICOVER™ installations is easy and allows the technician to safely inspect the production without opening the cover, which would risk contaminating the product. The VISIOCOVER™ Cam…
eBook: Advances in Single-Use Platforms for Commercial Manufacturing
This special report from Sartorius Stedim Biotech explores recent advances in single-use process platforms for commercial production of biopharmaceuticals. Enormous developments have been made in single-use systems, allowing biomanufacturers to adopt the technology in critical applications within their commercial-scale facilities. The authors illustrate how companies can work with teams of experts from supplier organizations to implement end-to-end single-use bioprocesses at scales up to 2,000 L by carefully considering the approach from the earliest stages in process development. Finally, the report…
Sartorius Stedim Biotech Launches SIMCA and SIMCA-online Software Updates
Sartorius Stedim Biotech (SSB) has introduced a new version of its SIMCA® and SIMCA®-online data analytical solutions, which are offered by its subsidiary Sartorius Stedim Data Analytics, formerly known as Umetrics. Every day, businesses generate a vast array of data derived from a variety of different sources. This data holds the key to better performance. The challenge is to interpret this information in a meaningful way. However, with so many parameters and such vast system complexity to consider, it is…
eBook Download: A New Paradigm in Process Development
As the need to accelerate biopharmaceutical development around the world continues to grow, biomanufacturers face a host of challenges so they, too, can grow. Increasing process productivity, reducing cost, mitigating risk, and bringing products to market faster are just a few of the issues frequently addressed. But with support in process development, cGMP manufacturing and training, accelerating bioprocess development can become less challenging. Several biomanufacturers have successfully navigated these issues in collaboration with GE Healthcare’s Fast Trak Services. Whether…