This webcast features: Lisa Krapf, Field Automation Scientist, Unchained Labs Thorough particle sizing and analysis are essential steps in formulation development and the monitoring of manufacturing process controls. Traditional particle analysis techniques such as light obscuration do not provide morphological information that can be useful for classification. Transfer to manufacturing and scaling to production will necessitate different equipment and the potential introduction of extrinsic particles that may not have been observed previously. The combination of particle analysis with flexible options…
Author Archives: BPI Contributor
Accelerate Cell and Gene Therapy Development and Manufacturing with Fully Integrated Closed CAR-T Cell Therapy Platform
For many years, the primary forms of cancer treatment have been chemotherapy, radiation, and surgery. An amazing breakthrough known as chimeric antigen receptor (CAR) T-cell therapy is being studied in the treatment of various types of cancer, including acute and chronic lymphoblastic leukemia, non-Hodgkin lymphoma, myeloma, and solid tumors. Developing innovative advanced therapies is one of our greatest opportunities to dramatically improve patients’ lives. WuXi Advanced Therapies recently announced the expansion of its service capabilities by offering a fully integrated…
Advantages of Custom Chromatography Resin Development: A Viable Solution for Purification of Complex Biotherapeutics
This webcast features: Scott Zobbi, Senior Manager of Business Development, Custom POROS Resins, Thermo Fisher Scientific Advances in biotherapeutic development are generating an increasing range of complex molecules, often presenting unique and complex purification challenges. Addressing these challenges requires novel purification strategies for commercial manufacturing, which may not always exist. When your specific process needs cannot be met with off-the-shelf resins, custom resin production may be a viable solution. Our POROS™ custom resin and CaptureSelect™ custom affinity matrix development platforms…
Protein or Not? Advanced High-Throughput Aggregate Analysis with the Aura™
This webcast features: Bernardo Cordovez, Chief Science Officer and Founder, Halo Labs In protein-based formulations, distinguishing aggregated active pharmaceutical ingredient (API) from other particle types is important for understanding the root cause of instability. Until now, existing methods have been either unreliable or too cumbersome to use in many workflows. Here we introduce the Aura™ 96-well low-volume aggregate and particle imaging system, which can rapidly size, count, and characterize particles and identify them as proteins, non-proteins, hydrophobic, or other types…
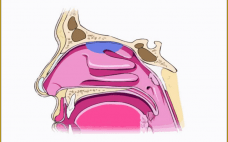
Translating Inhaled and Nasal Technologies for the Delivery of Biologics
This webcast features: Mark Parry, Technical Director, Intertek Inhaled and nasal delivery platforms have specific applications outside of their traditional uses for asthma/chronic obstructive pulmonary disease (COPD) and seasonal rhinitis/sinusitis: They can offer real advantages for the delivery of therapeutic biologics. During this short presentation, Intertek’s Technical Director, Mark Parry, will provide an overview of currently available technologies and successfully marketed products, with a look at the development challenges that might be encountered — and the solutions that are available…
Relative and Absolute Quantitation of Impurities and HCPs Using Mass Spectrometry
This webcast features: Steven Broome, Senior Mass Spectrometrist, BioPharmaSpec The processes involved in manufacturing a biopharmaceutical use biological and chemical systems to produce and purify the drug product. Therefore, the final active pharmaceutical ingredient (API) will often contain impurities related to these processes. It is a regulatory requirement to identify and monitor process-related impurities, and a qualitative and quantitative assessment of the components in the final drug product must be performed. Knowledge of the product-specific impurities, such as host-cell proteins…
Writing the Future of Biologics to Discover and Optimize Antibodies
With our unique DNA writing technology, Twist Biopharma is accelerating the way our partners discover and optimize antibody therapeutics. We have decades of synthetic antibody library design and selection expertise and have the ability to write any DNA sequence to enable faster, better discovery and development. Using our precise, rational, expertise in library fabrication, we can create libraries in a fundamentally different way that allow us to address tough targets, e.g. GPCRs. Since we are a DNA product company, we…
2.5× Lentiviral Vector Bioreactor Yield Increase and Simplified Execution with Innovative Tangential-Flow Depth Filtration
This webcast features: Michael Bransby, R&D Director of Process Technology, Repligen In a joint collaboration, Oxford Biomedica and Repligen increased the yield of viral vectors from suspension-cultured bioreactors several fold using tangential-flow depth filtration (TFDF). The yield for a single clarification step was 95% as compared to 70% by standard depth filtration. The TFDF tubular format and low shear enabled further yield increases through multiple harvests from the same seeding. A single seeded bioreactor produced two harvests of 95% and…
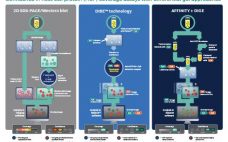
Confidence in Host Cell Protein (HCP) Coverage Assays with Differential Gel Approaches
Enzyme-linked immunosorbent assays (ELISAs) are critical to detecting and removing host cell proteins (HCPs), a primary source of impurities in biologics development. Scientists must validate HCP ELISAs to ensure patient safety and meet regulatory guidelines – but different coverage assays come with different challenges, limitations, and benefits. The US Pharmacopeia recommends 2D differential gel electrophoresis (DIGE) combined with Western blot or immunoaffinity approaches, and labs might not be able to gain the full benefits of DIGE without significantly altering current…
Optimizing Your Excipient Screening for Vaccine Formulation with an Ultra-Pure Pharmaceutical Gelatin
This webcast features: Jeroen Geeraerts, Business Development Manager, Biomedical, Rousselot The world is working at an unprecedented pace to develop a safe and effective vaccine to combat the COVID-19 pandemic. Currently, five candidate vaccines are in clinical evaluation, and many more are in preclinical testing. Different types of vaccines are being developed using multiple strategies and platforms. Among them are several inactivated-virus and live-attenuated–virus candidate vaccines. As an excipient, gelatin is a key component in many vaccine formulations. Well-known examples…