Recent advances in molecular biology are expediting genomic sequencing to underpin precision medicine. Such progress is positioning gene and gene-modified cell therapy on the cusp of an extraordinary revolution in patient care for presently unmet medical needs — and a new therapeutic class that could rival monoclonal antibodies (MAbs) in importance. However, despite substantial strides made in clinical trials, the bioprocessing community is struggling to fulfill growing demands for biomanufacturing capacity to make gene and gene-modified cell therapies — including…
Author Archives: James A. Smith
Automation of CAR-T Cell Adoptive Immunotherapy Bioprocessing: Technology Opportunities to Debottleneck Manufacturing
Continued clinical efficacy demonstrations of cell-based immunotherapies (iTx) such as chimeric antigen receptor T cell (CAR-T) therapies has made the prospect increasingly likely of an immunotherapy product achieving conditional market authorization in the short term. For example, Novartis and the University of Pennsylvania’s lead candidate (CTL019) for treating a range of hematological malignancies received breakthrough status from the US Food and Drug Administration (FDA) in 2014, permitting access to an expedited drug development pathway for high unmet medical needs (1).…
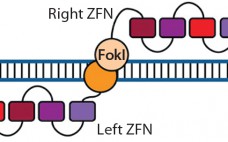
Therapeutic Gene Editing: Tools to Facilitate Basic Science or Stimuli for a Paradigm Shift in Biomanufacturing?
Historically, fundamental science and process engineering were separated by distinct vernaculars and a decade or more in the translation pathway of candidate therapeutics from laboratory to bedside (1). This crude metric holds true for the origins of the modern pharmaceutical industry, namely fine chemicals that supported the high-margin small molecules that constitute the majority of the pharmacopoeia even today. But as illustrated by deeply interwoven careers, companies, and technologies — including those related to monoclonal antibodies (MAbs) — that classic…
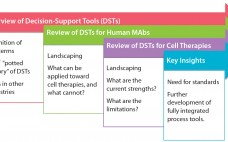
Decision-Support Tools for Monoclonal Antibody and Cell Therapy Bioprocessing: Current Landscape and Development Opportunities
Industrial-scale manufacturers in a number of fields — from automobiles to biotherapeutics — have long relied on powerful computational and mathematical tools to aid in the scale-up, optimization, quality control, and monitoring of product development (1–5). Typical process pathways are highly multifactorial, with numerous branch points, feedback steps, instrumental attributes, and target parameters. Moreover, margins for error are minimal for most industrial processes, requiring high standards of precision from industrial and operational pathways (6). For those reasons, the complexity of…
Quantitative Risk Assessment of Bioaccumulation Attributable to Extractables and Leachables in Cellular Immunotherapy Biomanufacturing
Precious patient samples, contamination concerns, and limited product purification options have compelled manufacturers of cellular immunotherapies (iTx) such as chimeric antigen receptor T cells (CAR-T) and T-cell receptor (TCR) technologies toward the disposables industry. Such companies are implementing single-use technologies (SUTs) almost exclusively (1). But despite the dominance of disposable bioprocess platforms and their extraordinary growth in the iTx marketplace, researchers have made limited efforts to understand the perennial and critical bioprocessing risks of leachables and extractables. Here we outline…
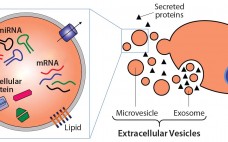
Extracellular Vesicles Commercial Potential As Byproducts of Cell Manufacturing for Research and Therapeutic Use
Extracellular vesicles (EVs) are emerging as a potential alternative to some stem-cell–derived therapeutics (1, 2). Sometimes called exosomes, they are small, secreted vesicles that can possess similar therapeutic mechanisms to whole cells, possibly representing the active pharmaceutical ingredient. In the past 15 years, academic and industry interest in EVs has exponentially increased as mounting evidence demonstrates their role in physiology and pathology as well as their therapeutic potential. In light of growing efforts in using EVs for research and therapy,…
The Potential Application of Real‑Time Release Testing for the Biomanufacture of Autologous Cell‑Based Immunotherapies
Cell-based immunotherapies (iTx) are emerging as a truly transformative therapeutic modality that is both complementary and convergent with existing regenerative medicine approaches, including gene therapy, cell therapy, and tissue engineering (Figure 1). Critically, iTx offer step-change improvements in efficacy compared with current standards of care (1) for a range of clinical indications and unmet therapeutic needs — particularly oncology. The clear efficacy of iTx is in contrast with some previous regenerative medicine approaches, including early mesenchymal stem cell (MSC) therapies…