Blow–fill–seal (BFS) technology has been recognized by the industry as an advanced aseptic solution (1–3). Catalent Pharma Solutions has been commercially supplying sterile BFS products to the pharmaceutical industry for decades, primarily in the respiratory and topical ophthalmic markets. Such product formulations range from simple solutions to emulsions with drug substances from classical small molecules to large complex proteins such as biologics. The company also has optimized BFS processes and its Advasept plastic container system for the manufacture of sterile…
Author Archives: Gregory Bleck
Compatibility Assessment of a Model Monoclonal Antibody Formulation in Glass and Blow–Fill–Seal Plastic Vials
PREPRINT October 2015 issue Blow–fill–seal (BFS) technology has been recognized by the industry as an advanced aseptic solution (1–3). Catalent Pharma Solutions has been commercially supplying sterile BFS products to the pharmaceutical industry for decades, primarily in the respiratory and topical ophthalmic markets. Such product formulations range from simple solutions to emulsions with drug substances from classical small molecules to large complex proteins such as biologics. The company also has optimized BFS processes and its Advasept plastic container system for…
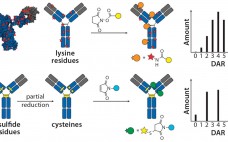
The Next Step in Homogenous Bioconjugate Development: Optimizing Payload Placement and Conjugate Composition
[Audio Recording] Bringing a new biologic drug to market is a long and expensive process, with research and development (R&D) cycles that can span up to 15 years and may cost over a billion dollars. Biologic drug development also involves significantly more complex manufacturing and CMC components than does development of small molecules. Nonetheless, the pharmaceutical industry is increasingly shifting its R&D efforts to focus on biologic drugs. According to a recent report from Tufts Center for Study of Drug…