Built on over 30 years of expertise in large-volume fluid processing with 3D single-use bags, Sartorius Stedim Biotech collaborated with many end users for the large-volume Flexsafe® 3D system with enhanced ergonomics, thereby bringing operator safety to the center of successful drug processing. We conducted in-depth interviews to gather and explore operator insights into the usability of large-volume single-use systems. That input helped us improve our product offering with the aim of also improving workplace health and well being. A…
Author Archives: Elisabeth Vachette
Outsourcing to Enhance Assurance of Supply: Application of Counterintuitive Supply Chain Strategies — A Case Study
Single-use technologies have transformed biopharmaceutical manufacturing by providing tremendous and proven opportunities to reduce costs, improve flexibility, and shorten cycle times. The expansion of such technologies into commercial production has naturally raised new challenges for both end users and suppliers, thus driving the need for a critical look at risks associated with their use. End users now face a new challenge: how to assess their own supply chains for robust assurance of supply. What is the suppliers’ responsibility in addressing…
Robust and Convenient Single-Use Processing: The Superior Strength and Flexibility of Flexsafe Bags
With the increased use of disposable bioprocessing bags in all critical process steps of the biopharmaceutical drug production, there is a growing requirement for high-quality, robust, and easy-to-handle bioprocessing bags. The new generation of films and bags must combine multiple mechanical, physical, and chemical properties to make these products suitable and scalable for all processing steps in upstream, downstream, and final filling operations, including cell culture in rocking motion and/or stirred-tank, single-use bioreactors as well as storage, mixing, shipping, and…
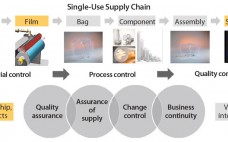
Enhanced Assurance of Supply for Single-Use Bags: Based on Material Science, Quality By Design, and Partnership with Suppliers
Growing adoption of single-use bags in commercial production of biopharmaceutical drugs raises new challenges for bag suppliers and drives the need for consistent product quality, improved assurance of supply, robust change management, and business continuity planning. In close collaboration with resin and film suppliers, polymer scientists and biologists at Sartorius Stedim Biotech have followed a stringent material science and quality by design (QbD) program to develop a completely new polyethylene film and to achieve consistent performance of new Flexsafe bags…