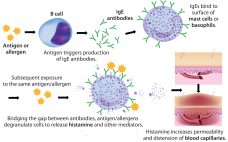
Rapidly increasing use of monoclonal antibodies (MAbs) in the treatment of neoplastic, autoimmune, and inflammatory diseases has led to a dramatic increase in hypersensitivity reactions worldwide, complicating the use of MAbs as first-line therapies and limiting patient survival and quality of life (1). The origins of anaphylaxis are not well understood, though its mechanism is fairly straightforward (Figure 1). It is usually attributed to some undefined intrinsic property or properties of a biotherapeutic β despite the fact that biotherapeutic formulations…
Author Archives: Edward Maggio
Alkyl Mono- and Diglucosides: Highly Effective, Nonionic Surfactant Replacements for Polysorbates in Biotherapeutics β a Review
Many biotherapeutic proteins are naturally subject to aggregation. The clinical consequences of protein aggregation can be dramatic, not only affecting bioavailability and pharmacokinetics, but in extreme cases dramatically altering pharmacodynamics as well. Of equal or perhaps more importance is that aggregation is a principal source of unwanted immunogenicity in biotherapeutics. Aggregation-induced neutralizing antibodies and/or anaphylactic reactions are serious and growing US and European regulatory concerns. So they will have significant and growing influence on the future development and regulatory approval…
Enhancing Antibody Production
Increasing demand for monoclonal antibodies (MAbs) — useful as immunodiagnostic reagents in basic research applications and potential immunotherapeutics — has created a need to optimize protein production techniques. Many developers have attempted to increase MAb output from cell culture by addressing both equipment and consumables. For example, recent advances in improved bioreactor designs and bioreactor operation have increased antibody outputs by increasing cell densities and extending cell life in culture. Materials and Methods () Bioreactors can operate in batch, perfusion,…
Biosimilars, Oxidative Damage, and Unwanted Immunogenicity
Concerns about the economic viability of biosimilars center on their high development cost relative to small-molecule generics, along with (and partly because of) the difficulty in demonstrating bioequivalence for these complex molecules. Immunogenicity is a particular area of increasing vigilance at both the US Food and Drug Administration (FDA) and the European Medicines Agency (EMA) (1, 2). Unwanted immunogenicity is an underlying cause of multiple deleterious effects for all protein-based therapeutics — including loss of efficacy, altered pharmacokinetics, and reduced…
Polysorbates, Immunogenicity, and the Totality of the Evidence
Protein aggregation underlies many deleterious effects for biotherapeutics. Principal among those are loss of efficacy, induction of unwanted immunogenicity, altered pharmacokinetics, and reduced shelf life. Consequently, aggregation-preventing surfactants are essential components of many protein formulations. They facilitate the development, manufacture, and stability of dosage forms by helping formulators manage protein aggregation and reduce interactions with container and delivery device surfaces. Monoclonal antibodies (MAbs) present difficulties with respect to aggregation because they usually require relatively high doses for therapeutic…