[Audio Recording] Bringing a new biologic drug to market is a long and expensive process, with research and development (R&D) cycles that can span up to 15 years and may cost over a billion dollars. Biologic drug development also involves significantly more complex manufacturing and CMC components than does development of small molecules. Nonetheless, the pharmaceutical industry is increasingly shifting its R&D efforts to focus on biologic drugs. According to a recent report from Tufts Center for Study of Drug…
Author Archives: David Rabuka
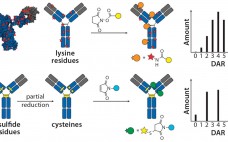
The Future Directions of ADC Development
This podcast features: David Rabuka, PhD, President, Chief Scientific Officer, Founder, Redwood Bioscience This podcast was recorded based on a presentation given at the 2014 BioProcess Theater which took place at the 2014 BIO International Convention, June 23-26, 2014. The BioProcess Theater, a 50-seat amphitheater located on the exhibition floor at the heart of the BioProcess Zone, provided attendees with daily, complimentary opportunities to listen, learn, and interact with leading experts as they presented, discussed and debated the latest business…