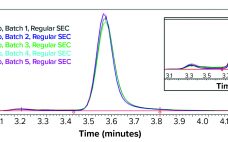
Therapeutic monoclonal antibodies (MAbs) mostly are manufactured using bioengineered mammalian cells cultured in a bioreactor for two to three weeks. High temperatures and an altered redox environment may compromise the quality of MAbs produced (e.g., fragmentation, truncation), as can the presence of proteases, reductases, and other chemicals released from dead cells. Thus, it would be valuable to establish analytical methods that can help cell culture groups monitor immunoglobulin G (IgG) product integrity in real time during a bioreactor run, especially…
Author Archives: Dharmesh Kanani
eBook: Addressing Quality in Cell-Line Development — Direct Analysis of Bioreactor Harvest for Clone Selection and Process Optimization
Using Direct Analysis of Bioreactor Harvest for Clone Selection and Process Optimization Therapeutic monoclonal antibodies (MAbs) mostly are manufactured using bioengineered mammalian cells cultured in a bioreactor for two to three weeks. High temperatures and an altered redox environment may compromise the quality of MAbs produced (e.g., fragmentation, truncation), as can the presence of proteases, reductases, and other chemicals released from dead cells. Thus, it would be valuable to establish analytical methods that can help cell culture groups monitor immunoglobulin…
NatriFlo® HD-Q Membrane Adsorber: A New Single-Use High-Capacity Polishing Tool with Excellent Salt Tolerance and Process Robustness for MAb Purification
Strong anion exchange (Q) chromatography has become an industry standard in MAb production. It is a proven technology to remove DNA, viruses, endotoxins and acidic host cell proteins from process feed streams in flowthrough mode. Recent trends show an increasing interest in downstream single-use technologies and flexible biomanufacturing due to advancement in cell culture technology and emergence of biosimilars. Traditional chromatography columns are slow, often oversized and not suitable for flexible biomanufacturing. Conventional membrane adsorbers cannot provide sufficient process robustness…