CRISPR engineered mammalian cell lines are more cost effective than other gene editing platforms says Oxford Genetics, but adopting such tech is perceived a risk due to ongoing patent issues. Oxford, UK-based synthetic biology firm Oxford Genetics has inked a multi-billion-dollar deal with an undisclosed ecommerce provider of reagents and tools for its high throughput automated genomic engineering platform for CRISPR modification of mammalian cell lines. While details are undisclosed, the deal shows how the firm has harnessed the gene-editing…
Author Archives: Dan Stanton
Ex-Shire director to aid Catalentâs shift to commercial manufacturing
The new general manager at Catalentâs facility in Wisconsin says his experience at Baxter and Shire will help the plant transition towards commercial manufacturing. Contract development and manufacturing organization (CDMO) Catalent has appointed Graham Brearley as general manager of its Madison, Wisconsin facility. With over 25 yearsâ of technical, operations and business experience in the biopharmaceutical industry, BioProcess Insider spoke to him about his move to a third-party services firm and his vision for Madison â and Catalent â going…
Jazz scales back Erwinaze push due to manufacturing disruptions
To minimize impact on patients, Jazz Pharmaceuticals has reduced promotion of its cancer drug due to continued manufacturing issues at its CMO Porton Biopharma Limited (PBL). Erwinaze (asparaginase Erwinia chrysanthemi), an asparaginase enzyme derived from the bacteria Erwinia chrysanthemi, is used for the treatment of patients with acute lymphoblastic leukemia (ALL) who have developed hypersensitivity to E. coli-derived asparaginase. The product is made for Jazz Pharmaceuticals by Wiltshire, UK-based and UK-government backed biomanufacturer PBL. However, the US Food and Drug…
YMC M&A: Lewa addition brings bioprocess tech and US market
Japanese separations resins firm YMC will buy the bioprocessing business of Lewa-Nikkiso America. The âfriendly acquisitionâ includes a facility in Devens, Massachusetts. Privately-held Kyoto, Tokyo-based YMC will add a number of bioprocessing technologies through the acquisition, including staff and assets dedicated to the development, manufacture and sales of batch and continuous chromatography equipment, and buffer dilution systems for the biopharmaceutical market. Financial details have not been divulged. Gerard Gach, chief marketing officer at Lewa- Nikkiso America, told BioProcess Insider Lewaâs…
Samsung Bioepis: From biosimilars to novel biologics
Following success with several biosimilars, bringing a novel biologic into the clinic is the ânext logical stepâ in becoming a fully integrated biopharma firm, says Samsung Bioepis. Almost a year to the date it announced a co-development agreement, Samsung Bioepis and Takeda Pharmaceuticals are preparing to begin a Phase I trial for its lead candidate, SB26, also known as TAK-671. The news signals the latest stage in the development of Samsung Bioepis â a joint venture between South Korean contract…
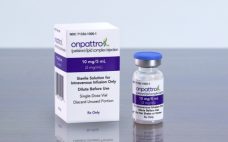
Alnylamâs success brings first RNAi therapeutic to US
The US Food and Drug Administration (FDA) has approved the first ever RNA interference (RNAi) therapy: Alnylamâs Onpattro (patisiran). Alnylam announced last week Onpattro has been approved in the US for the treatment of the polyneuropathy of hereditary transthyretin-mediated (hATTR) amyloidosis in adults. The product was hailed by the FDA as the first in a new class of drugs: small interfering ribonucleic acid (siRNA) treatments. âNew technologies like RNA inhibitors, that alter the genetic drivers of a disease, have the…
MilliporeSigma partners Innocore for sustained release tech
MilliporeSigma will offer customers access to a sustained release formulation platform for large molecule APIs through a partnership with Innocore. The bioprocessing equipment and tools subsidiary of Germanyâs Merck KGaA, MilliporeSigma, has inked an agreement to be the partner for the introduction of Innocureâs SynBiosys biodegradable platform to the global biopharma market. The platform can be used to develop sustained release solutions for biologicals in injectable formulations, something MilliporeSigma said is becoming increasingly important and competitively tempting for biopharmaceutical developers.…
Celltrion receives FDA 483 with 8 âmanageable and correctibleâ observations
Celltrion has received a US FDA Form 483 at its troubled biomanufacturing plant in Korea with eight observations not raised in a previous warning letter. In January, the US Food and Drug Administration (FDA) sent Korean drugmaker Celltrion a warning letterâ highlighting âmultiple poor aseptic practicesâ at its production site in Songdo, Incheon. An FDA reinspection last month has now resulted in a Form 483 with eight observations. The 483 itself, uploaded by the Agency, cites issues with Celltrionâs drug product and…
Anti-antibody anybody? Bio-Rad range to target Lucentis biosimilar developers
Bio-Rad has launched a range of anti-ranibizumab antibodies for PK and immunogenicity assays on the back of increased Lucentis biosimilar development. Bio-Rad Laboratories will offer customers a range of recombinant monoclonal anti-ranibizumab antibodies that are highly specific for Lucentis (ranibizumab), or the complex of ranibizumab with its target, vascular endothelial growth factor A (VEGF-A). The anti-antibodies are to be used in pharmacokinetic (PK) and immunogenicity assays, and the product launch is due to the increased interest in Lucentis biosimilar development,…
Repligen riding high on Protein A strategic partnerships
Repligen says strategic partnerships are strengthening its proteins business, following a quarter which saw deals inked with Navigo Proteins and Purolite. For the second quarter 2018, bioprocessing tools and equipment supplier Repligen Corporation reported total revenues of US$48 million (âŹ42 million), up 47% on the same period last year. The firm also reported sequential gains in its proteins franchise, where revenue increased nearly 10% over the first quarter. The segment includes its Protein A chromatography ligand and cell culture capture…