Do not confuse the methods and purposes for controlling the serum supply chain by misleading end-users into believing that some origins of serum are better and safer than others, says the European Serum Products Association (ESPA). Last month, Bioprocess Insider published an article entitled: ‚ÄėQuality FBS, or just BS? Industry turning to supply chain certification‚Äô. The article detailed a presentation from the International Serum Industry Association (ISIA) at BPI Europe in Vienna, Austria and highlighted the application of independent audits,…
Author Archives: Dan Stanton
Partnerships abet vendors in tackling cell & gene logistics and automation
With Thermo Fisher teaming with automated tech company Scinogy and GE Healthcare collaborating with logistics firm World Courier, both vendors have increased their advanced therapy services. The recent approval of Novartis‚Äô one-off gene therapy Zolgensma (onasemnogene abeparvovec) has cemented the advent of advanced therapies. With hundreds more cell and gene therapies in development, the US FDA has predicted that up to 20 such products will be approved each year by 2025. Vendors, therefore, have been increasing their commercial services technologies…
Zolgensma approval marks success for REGENXBIO’s vector platform
REGENXBIO could receive up to $80 million after the US FDA approved Novartis/AveXis‚Äô gene therapy Zolgensma (onasemnogene abeparvovec), which uses the firm‚Äôs NAV Technology Platform. Last week, the US Food and Drug Administration (FDA) approved Zolgensma, a single-dose gene therapy developed by Novartis acquisition AveXis, for the treatment of children less than two years of age with spinal muscular atrophy (SMA). The approval triggered a $3.5 million (‚ā¨3.1 million) milestone payment to Maryland-based biotech REGENXBIO. Zolgensma is based on REGENXBIO’s…
Bavarian Nordic considers turning CMO as it builds out network
‚ÄúHaving manufacturing capabilities is both a blessing and a noose around your neck,‚ÄĚ says Bavarian Nordic as it considers CMO opportunities. Bavarian Nordic has expanded its manufacturing footprint over the past few years, including expanding its bulk vaccine capabilities and adding a fill/finish facility in Kvistgaard, Denmark. The $75 million fill/finish plant is on track to be finalized by the end of the year, with production expected to begin in 2021. The growth of its manufacturing network will support its…
Personalized medicines need personalized pricing plans, Orchard
There is no one-size-fits-all model for cell and gene therapy pricing plans says Orchard Therapeutics, but industry must adapt a system set up for chronic care to incorporate curative one-off treatments. There have been questions over how payors and insurance firms would cope with such next-generation therapeutics have been asked ever since Novartis launched its first cell and gene therapy product Kymriah (tisagenlecleucel) in 2017 at a list price of $475,000. But the recent US Food and Drug Administration (FDA)…
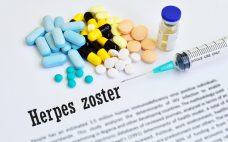
Will GSK have the capacity to bring Shingrix to China?
GlaxoSmithKline (GSK) has received Chinese approval for its shingles vaccine Shingrix, but with demand already outstripping supply the firm must invest significantly in its production capabilities. China‚Äôs National Medical Products Administration (NMPA) has approved Shingrix, a non-live, recombinant subunit vaccine for the prevention of shingles (herpes zoster) in adults aged over 50. Shingrix underwent an expedited review after falling on a list of 48 ‚Äėclinically urgently needed new medicines‚Äô in China aimed at bringing critical new prevention and treatment options…
Novartis wins approval for $2.1m gene therapy Zolgensma
Novartis says it is ready to launch Zolgensma (onasemnogene abeparvovec) within the next few weeks after receiving FDA approval for its spinal muscular atrophy (SMA) single-dose gene therapy. Zolgensma, a single-dose, one-time gene therapy has been approved to treat children less than two years of age with spinal muscular atrophy (SMA). ‚ÄúToday‚Äôs approval marks another milestone in the transformational power of gene and cell therapies to treat a wide range of diseases,‚ÄĚ acting US Food and Drug Administration (FDA) commissioner…
ProBioGen licenses duck cell line tech to Vaccitech for viral vaccines
Vaccitech has licensed the AGE1.CR duck retina cell line from ProBioGen to manufacture its viral vectored vaccines. The deal will see University of Oxford‚Äôs Jenner Institute spin-out Vaccitech gain access to ProBioGen‚Äôs technology platform based on the AGE1.CR duck retina cell line for production of its viral vectored universal flu vaccine. ‚ÄúWe generated the AGE1.CR cell line from an embryonated Muscovy duck egg,‚ÄĚ Ingo Jordan, director of Vaccine Strategies at ProBioGen told Bioprocess Insider. This, he explained, makes it well…
FDA issues draft guidance on biosimilar analytical studies
The draft guidance advises industry to minimize differences between a biosimilar and its reference biologic including in the choice of expression system and manufacturing process. The US Food and Drug Administration (FDA) has been active in encouraging biosimilar development of late, publishing its long-awaited interchangeability guidance earlier this month. In its latest push the Agency has issued draft guidance entitled: ‚ÄėDevelopment of Therapeutic Protein Biosimilars: Comparative Analytical Assessment and Other Quality-Related Considerations Guidance for Industry.‚Äô The document makes recommendations on…
Off-the-shelf CAR-T a ‚Äėgamechanger‚Äô for multiple myeloma, says Allogene
Allogene expects manufacturing specifications to be a focus of IND reviews as it looks to bring an off-the-shelf CAR-T therapy for multiple myeloma through the clinic. Chimeric antigen receptor (CAR) T-cell therapies have been described as ‚Äúone of the newest, emerging weapons in the fight‚ÄĚ against multiple myeloma, a cancer affecting around 300,000 Americans every year. Some of the candidates in the clinic for multiple myeloma include Bluebird and Celgene‚Äôs BB21217, Celgene‚Äôs JCARH125 and Janssen/Legend Biotech‚Äôs LCAR-B38M, but these ‚Äď…