Biocon says it is making a ‚Äúvery significant expansion‚ÄĚ in its manufacturing capacity to feed the demand for biosimilars, including its pegfilgrastim product Fulphila. In June 2018, Fulphila became the first biosimilar of Amgen‚Äôs cancer drug Neulasta (pegfilgrastim) to win US Food and Drug Administration (FDA) approval. The biosimilar is marketed in the region by Mylan but manufactured by partner Biocon. In its Q1 FY2020 results, the Indian drugmaker claimed Fulphila captured a 21% volume share of the US pegfilgrastim…
Author Archives: Dan Stanton
GE: Learning from MAb automation to tackle CAR-T
GE Healthcare says monoclonal antibodies laid the groundwork for its workflow technologies, allowing accelerated uptake of automation in the autologous cell and gene therapy space. In May, bioprocessing tools and services firm GE Healthcare launched its Chronicle automation software for cell therapy manufacturing. The offering supports the complete workflow for cell therapy products including the optimization of manufacturing from process development to commercialization. Talking to Bioprocess Insider at the BPI Theater at BIO in June, Catarina Flyborg, GM of GE‚Äôs…
On the biotech doorstep: Why Sartorius moved its bioanalytics to Cambridge
Sartorius Stedim Biotech says it consolidated its cell line and testing solutions in Cambridge, Massachusetts to be close to the industry it serves. Services like analytics, QC and characterization, have traditionally been done inhouse. ‚ÄúIt was done by what were referred to at one time as testing houses. These were mostly very large QC labs that focused on QC release for firstly traditional pharma products, but that migrated to the biologics,‚ÄĚ Maurice Phelan, site head of Cambridge US, Sartorius Stedim…
Danaher: ‚ÄėAll indications show GE is in wonderful shape‚Äô
Danaher Corporation says customers are excited about the combination of Pall Biotech and GE Biopharma as it moves closer to completing the $21.4 billion acquisition. In February, Danaher announced it had reached an agreement to acquire the biopharma division of GE Healthcare for $21.4 billion (‚ā¨19.1 billion). Danaher first entered the bioprocess space through its $13.8 billion acquisition of Pall Corporation¬†in 2015. This acquisition will, therefore, make Danaher the largest vendor with a fully end-to-end bioprocessing offering. The deal is…
Big Pharma M&A a boon for business, says Thermo Fisher
Thermo Fisher says it is well positioned to benefit from recent M&A activity within the biopharma space: Takeda and Shire, BMS and Celgene, AbbVie and Amgen. So far, 2019 has been a big year for Big Pharma megamergers. Takeda completed its $62 billion (‚ā¨56 billion) takeover of Shire in January, Bristol-Myers Squibb is moving closer to joining with Celgene in a $74 billion deal, and last month AbbVie entered an agreement to buy Allergan for $63 billion. The biopharma space…
Germany‚Äôs Merck fully prepped for a ‚Äėworst-case scenario‚Äô Brexit
Merck Life Science says it has undergone Brexit preparations to limit supply chain issues if the UK leaves the European Union without a deal in place. In 2016, a referendum in the UK resulted in a slight majority of an ill-informed electorate to vote to leave the European Union (EU). Three years on and with no agreement in place as it stands, the UK is set to leave the bloc and the single-market economy on October 31 without any arrangements…
Shingrix a ‚Äėstandout‚Äô in GSK‚Äôs vaccine strong Q2
GlaxoSmithKline (GSK) has described its shingles vaccine Shingrix as a driving force in its Q2 sales results. The firm is expanding capacity to keep up with demand. For the second quarter 2019, group sales stood at ¬£7.8 billion ($9.8 billion), up 7% year-on-year. GSK saw a 1% decline to ¬£4.3 billion in its pharmaceuticals division but was boosted by strong performances in vaccines and consumer. Management attributed vaccine sales of ¬£1.6 billion ‚Äď up 23% on the same period last…
Garbage in, garbage out: Raw material quality crucial for cell & gene therapies
Cell and gene therapy makers have suffered manufacturing setbacks, but experts working in the supply chain say ensuring high quality raw material is the key to success. Manufacturing issues have plagued the few advanced therapies to have made it to the market. Dendreon‚Äôs Provenge (sipuleucel-T) suffered from high complexity of manufacturing and administration, Novartis‚Äô Kymriah (tisagenlecleucel) saw some variability in its product specifications, and ‚Äď most recently ‚Äď Bluebird Bio has been accused of lacking manufacturing readiness for its recently…
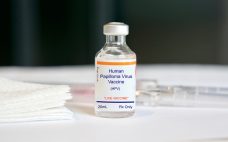
Merck builds up Gardasil network again with $680m NC injection
Merck & Co. will build a 225,000 square-foot facility in Durham to support production of its HPV vaccine Gardasil, creating 400 jobs. Merck will report its second quarter 2019 results on July 30, but for the previous quarter the firm saw a 31% year-on-year increase in sales of its human papillomavirus (HPV) vaccines Gardasil and Gardasil 9, which pulled in sales of $838 million (‚ā¨752 million). Adam Schechter, Merck‚Äôs president of Global Human Health, said last year that his firm…
Alexion on track with FcRn antibody after manufacturing hitch
After inheriting an impurity in the drug product of early-phase antibody ALXN1830, Alexion Pharmaceuticals says new batches will be available by the end of the year. In November 2018, Alexion Pharmaceuticals acquired Syntimmune in a deal worth up to $1.2 billion, adding ALXN1830, a clinical-stage humanized monoclonal antibody that inhibits the interaction of neonatal Fc receptor (FcRn) in trials for warm autoimmune hemolytic anemia (WAIHA) and generalized myasthenia gravis (gMG). But earlier this year, the firm discovered an issue in…