Cytiva has upgraded its Fast Trak facility in Marlborough in response to demand for pre-clinical to Phase II biomanufacturing services from small to mid-sized companies. Under the firm’s Fast Trak contract development and manufacturing brand, Cytiva has expanded its Marlborough cGMP facility to 60,000 square feet. The plant offers biopharma access to services from preclinical to Phase II, including process development, clinical batch manufacturing, analytical development, and QA/QC/regulatory support. Olivier Loeillot, senior vice president of BioProcess at Cytiva, did not…
Author Archives: Dan Stanton
Nex-T up: Bristol-Myers using machine learning to optimize future CAR-T
Bristol-Myers Squibb says its next-generation CAR-T candidates, known as Nex-T, could offer faster and cheaper autologous cell therapies against multiple myeloma and lymphoma. Bristol-Myers Squibb (BMS) entered the chimeric antigen receptor (CAR) T-cell therapy space through the completion of its $74 billion acquisition of Celgene last November. Among the candidates BMS added to its pipeline were lisocabtagene maraleucel (liso-cel), an anti-CD19 targeting lymphoma, and idecabtagene vicleuce (ide-cel) being developed against multiple myeloma in partnership with Bluebird Bio, both in late-stage…
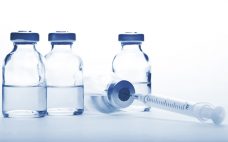
COVID-19 vaccine rush magnifying glass demand, says Stevanato Group
Gerresheimer, The Stevanato Group, and SCHOTT have pledged to safeguard the supply of glass vials as demand from COVID-19 related projects gets set to skyrocket. All parts of the biopharma supply chain will be called upon to support the increasing numbers of COVID-19 vaccines entering and progressing through the clinic. As such, three glass and primary packaging firms have come together to issue a statement promising to work together and with industry to ensure supply of such elements throughout all…
Imperial College London takes RNA COVID vaccine into clinic
A vaccine candidate that allegedly turns cells in the body into ‘mini-antibody producing factories’ against COVID-19 has begun first-in-human clinical trials. The latest effort in combatting COVID-19 comes form the UK’s Imperial College London, with its vaccine candidate beginning Phase I trials this week. The vaccine is being developed through over £41 million ($51 million) of UK government funds and is based on a self-amplifying RNA technology. “The way the vaccine works is quite different from other approaches in that…
Inhouse and out: AstraZeneca secures COVID-19 vaccine capacity
Contracts with CDMOs Catalent, Emergent Biosolutions, Cobra, and Novasep and the retrofitting of a facility in Ohio will support AstraZeneca and the Oxford University’s COVID-19 vaccine candidate. AstraZeneca was among the second wave of Big Pharma firms to enter the rush to develop a vaccine against the coronavirus SARS-CoV-2 that causes COVID-19 in April when it teamed with the Jenner Institute and the Oxford Vaccine Group at the University of Oxford on its ChAdOx1 nCoV-19 program. Production of the vaccine…
Samsung Biologics selects San Francisco for first US facility
Korean CDMO Samsung Biologics will open an R&D lab in the San Francisco area later this year, though admits to delays due to COVID-19. Contract development and manufacturing organization (CDMO) Samsung Biologics has established itself as a large-scale biologics producer from its base in Incheon, South Korea. Along with 364,000 L of bioreactor capacity, the firm expanded its services in 2017 to include cell line and process development (PD) services. And now the firm is coming to the US. Eagle-eyed…
Spark on changing cell and gene regulatory expectations
Regulators are increasingly looking to industry to turn the science of cell and gene therapies into reproducible platform technologies, says Spark Therapeutics’ Diane Blumenthal. The advancements in the cell and gene therapy space were applauded throughout Xconomy’s virtual Xcelerating Philadelphia event last night as industry experts spoke about the increasing number of such therapies moving through the clinic and the hurdles that industry is rapidly knocking over. But challenges continue to exist, and in a panel focused on regulatory pathways,…
Gilead’s Kite receives EMA OK for Amsterdam CAR-T plant
The $150 million facility in The Netherlands is now fully operational and will produce CAR-T therapy Yescarta for up to 4,000 patients per year, says Kite. In 2018, Gilead Sciences, fresh from its $11.9 billion acquisition of Kite Pharma, announced plans to open a European facility to manufacture chimeric antigen receptor (CAR) T-cell therapies. Today the firm has announced the 204,514 square-foot site – the largest in Kite’s network – has received approval from the European Medicine Agency (EMA) for…
Is Catalent set to make another major acquisition?
With CDMO Catalent set to raise around $550 million in a public offering, some commentators believe another large acquisition could be around the corner. Contract development and manufacturing organization (CDMO) Catalent has announced the pricing of a public offering of shares of its common stock, expected to close next week resulting in proceeds of approximately $550 million. This is the third time in two years that the firm has tapped the public equity market. In July 2018, the CDMO announced…
Rigenerand launches CDMO biz after gene therapy facility gains approval
Regulatory approval to make gene therapies from its site in Modena, Italy has enabled biotech Rigenerand SRL to enter the advanced therapy CDMO space. Rigenerand announced last week that the Italian Medicine Authority (AIFA) has granted authorization to produce gene therapy medicinal products for clinical purposes from its site in Modena. This will allow the advancement of the firm’s lead candidate RR001 – an autologous gene therapy being investigated to treat pancreatic cancer – into Phase I trials but will…