Clene Nanomedicine has quadrupled production capacity to support candidate CNM-Au8, a gold nanocrystal suspension it says could be a gamechanger in the neurodegenerative disease space. The Utah-based biotech has entered into both a 10 year lease for a manufacturing space located in Elkton, Maryland and a seven year lease to further expand its existing manufacturing capacity nearby. The expanded capabilities will serve to support lead candidate CNM-Au8, currently in Phase II studies for amyotrophic lateral sclerosis (ALS). “In our existing…
Author Archives: Dan Stanton
COVID-19 boosters set to bolster life sciences services firms
With booster shots on the agenda, large life sciences vendors and contract manufacturers are likely to see the COVID-19 windfall continue for the foreseeable future. It has only been nine months since vaccines began being rolled out to combat COVID-19, but some countries are now administering, or planning to administer, third shots to vulnerable patients. Nearly a million Americans have already received a booster shot, while a third dose of Pfizer/BioNTech’s vaccine is becoming part and parcel of Israel’s immunization…
Seqirus looks to next-gen mRNA for seasonal flu vaccine
Seqirus says it will look to build large-scale manufacturing capacity to support its self-amplifying messenger RNA (sa-mRNA) ambitions in the seasonal influenza space. Seqirus has long had a leading role in the seasonal influenza space through its portfolio of products, including both egg-based and cell-based vaccine. But now the firm is stepping up its efforts to develop a flu vaccine based on mRNA, launching a dedicated program to support development of its next-generation sa-mRNA platform with plans to set up…
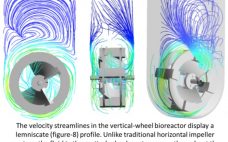
PBS to expand single-use bioreactor tech to feed cell therapy demand
Single-use bioreactor maker PBS Biotech has closed a private $10 million funding round with BroadOak Capital Partners to advance its Vertical-Wheel technology. The funding will be used to expand PBS’ internal operations and capacity, the firm told Bioprocess Insider, to meet the demand and need for its biomanufacturing customers, specifically for cell therapies. Such “investment in manufacturing, engineering and contract services capabilities is required to maintain our rapid growth trajectory in the cell therapy market, we were told. The California-headquartered…
eBook: Cell and Gene Therapies —
A 2021 Industry Update
The US Food and Drug Administration (FDA) reports that as of June 2021, 22 advanced therapy products have received regulatory approval in the United States. The first such product gained regulatory approval in 2010. Since then, hundreds of cell and gene therapies have advanced to clinical evaluation, but few products have reached commercial stages — and those that have done so have been hindered by manufacturing problems. In this eBook, writers from the BioProcess Insider and Project Farma analyze trends…
EMA thumbs up to more CDMO sites supporting COVID vaccines
The European Medicines Agency (EMA) has approved a Delpharm plant in France and a Catalent facility in the US to support supply of Pfizer and Moderna’s COVID-19 vaccines, respectively. The approvals from the EMA’s human medicines committee (CHMP) this week aim to increase manufacturing capacity and supply of the two COVID-19 vaccines in the EU. The first saw the Agency approve a fill-finish site in Saint Remy sur Avre, France operated by contract development and manufacturing organization (CDMO) Delpharm. The…
WuXi’s ongoing capacity drive ups planned MA investment to $300m
WuXi Biologics is expanding bioreactor capacity at its site in Worcester, Massachusetts from 6,000 L to 24,000 L to support demand for biomanufacturing onshoring. Plans for a first US facility were drawn up by China-headquartered contract development and manufacturing organization (CDMO) WuXi Biologics in 2018, with construction beginning in Worcester two years later. But after winning planning approval from the local council last week, WuXi Biologics will expand the site, quadrupling bioreactor single-use capacity through an additional $235 million of…
COVID continues to impact biopharma supply chains, survey
Industry continues to feel the effect of supply chain constraints caused by the COVID-19 pandemic, according to the latest industry survey from Informa Connect. The Delta variant. Booster shots. Vaccination hesitancy. Mask mandates (or lack of them). The coronavirus pandemic is clearly still with us, 18 months on from the first reports of a new virus coming out of Wuhan, China. And it continues to affect the life sciences industry, according to Informa Connect’s latest survey. ‘COVID-19 One Year On:…
M&A rife in manufacturing space, driven by big CDMOs and PE
The CDMO space is perceived as fragmented and particularly lucrative says GlobalData, with private equity (PE) firms and large established players investing heavily in the sector. A report from data and analytics company GlobalData entitled ‘M&A in the Contract Manufacturing Industry: Implications and Outlook – 2021 Edition’ has found that dealmaking in the sector has been particularly rife over the past few years, predominantly led by PE firms and big contract manufacturing organizations (CMOs). “Investors perceive the CMO industry as…
Lilly hit by FDA 483 with 7 observations at IN plant
An Eli Lilly aseptic facility in Indianapolis, which fills COVID-19 antibodies along with other drugs, has received a US FDA Form 483 with seven observations. The Form 483 (available below) was published last week and highlights the issues the US Food and Drug Administration (FDA) found after inspecting the facility in Indianapolis, Indiana in February and March this year. Among the seven observations noted, the Agency highlighted failures by Lilly to establish an adequate system for monitoring environmental conditions in…