Quantitation of different capsid species in viral-vector gene-delivery drugs, including recombinant adenoassociated virus (AAV) therapies, is essential for proper assessment of critical quality attributes before regulatory approval and during commercial production. If rAAV full-capsid percentages are maximized, and variants such as empty or partially-filled capsids are reduced as much as possible, then potency is increased for improved dosing and tolerance outcomes. Traditionally, characterization of AAV purity with respect to empty, full, and other variants has been performed by techniques such…
Author Archives: Christopher Sucato
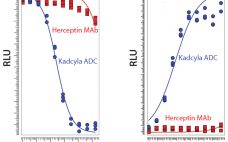
Biological Stealth Bombers: Potency, Regulatory, and Bioprocessing Concerns of Antibody–Drug Conjugates
Seven years ago, the US Food and Drug Administration (FDA) approved the first product in a new class of biologics: antibody–drug conjugates (ADCs). The idea for these products already had been hatched a decade earlier when the promising field of antibody research — touting such molecules as “magic bullets” — had faltered, specifically against oncology-related indications. The early crop of anticancer monoclonal antibodies (MAbs) proved to have only limited efficacy, and interest in developing antibodies as therapeutic agents against cancer…