Plato wrote in ancient Greece that “our need will be the real creator,” which transformed over time into the English proverb, “Necessity is the mother of invention.” Advancements in medicine and biomanufacturing technology in 2020 have epitomized that idea. Even as technologies such as mRNA vaccines have rocketed into the public’s awareness, biomanufacturing experts have worked behind the scenes with renewed vigor spurred on by hard lessons from the pandemic. Cell-line development and engineering are no exception. Already undergoing a…
Author Archives: Cheryl Scott
Increasing Expression Titers: New Technologies Could Help Other Cell Lines Catch Up to CHO
Fang Tian is a lead scientist and head of cell biology research and development at the American Type Culture Collection (ATCC) in Manassas, VA. She is a member of both the International Cell Line Authentication Committee (ICLAC) and the US technical advisory group for the ISO/TC276 technical committee. At ATCC, she oversees preparation, authentication, characterization, quality control, and cryopreservation of more than 3,400 accessioned animal cell lines and hybridomas in the cell biology general collection. She holds a PhD in…
Engineering Alternatives: Modern Technology Enables Expression System Developers to Think Beyond CHO Cells
Major biopharmaceutical companies are teaming up with academics and the Bill & Melinda Gates Foundation to develop new biomanufacturing cell lines and methods. The project — known as the AltHost Consortium — is exploring innovative ways to produce biologics and vaccines for clinical usage in diseases from diabetes to cancer. Lead researcher J. Christopher Love at the Massachusetts Institute of Technology (MIT) likens this precompetitive, open-access collaboration to the early days of the biopharmaceutical industry. “When biomanufacturing first emerged as…
Cell-Free Expression: A Technology with Truly Disruptive Potential
Bioprocess engineer Beatrice Melinek is a postdoctoral research fellow at University College London’s Future Targeted Healthcare Manufacturing (FTHM) Hub, where she focuses on the use of cell-free protein synthesis (CFPS) as a platform for distributed production of stratified biotherapeutics. Previously Melinek specialized in purification of viral vectors and vaccines, with an engineering doctorate (EngD) in biochemical engineering and postdoctoral experience in UCL’s hematology department developing a new chromatography-based analytical method for measuring empty and full adenoassociated virus (AAV) capsids. She…
eBook: mRNA — Revisiting a Technology That Has Rocketed into Success
At the end of 2018, BPI published its first eBook about mRNA drug products — and quite a lot has happened since then! Our initial report highlighted companies working on mRNA therapeutics for cystic fibrosis, heart disease, and cancer, as well as vaccines. The latter approach took off in 2020 with the advent of SARS-CoV-2 and the COVID-19 pandemic, and in a stunningly short time, the biopharmaceutical industry has learned much about manufacture, formulation, product design, and distribution of mRNA…
April 2021: From the Editor
I write this the day after US President Joe Biden’s address reporting his administration’s progress on COVID-19. Vaccines and their distribution and delivery are priorities, of course, and the companies working on them over the past year deservedly have received a great deal of attention. But our pandemic response rests on three other important pillars as well: prevention of exposure through masking and social distancing (as the President reminded us), testing and tracing with diagnostics and data, and treatment of…
Introduction: Technologies Converge in Biopharmaceutical Laboratories
Bioassay development is foundational to the well-characterized biotechnology product paradigm. Bioassays are the best tools for drug developers to use in determining the biological activity (potency) of their products, which has been a biopharmaceutical critical quality attribute (CQA) since long before that concept had a name. Thus, these assays are vital to quality assurance and quality control (QA/QC), preclinical studies, and clinical testing — and by extension to process development and monitoring. Because of their complex nature, bioassays are among…
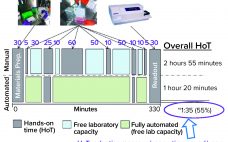
Automation of Potency Assays: A Strategic Journey
Cell-based potency testing provides quantitative data concerning a drug’s biological activity. Thus, it plays an essential role in biopharmaceutical quality control (QC), good manufacturing practice (GMP) product release, comparability determination, and stability testing for both drug products and drug substances. Potency is a critical quality attribute (CQA) often scrutinized by regulators and reviewers. Test methods are specific to a drug’s mechanism of action (MoA) and should be validated to internationally harmonized regulatory standards (1). The options preclude applying a simple…
HCP Assay Development: Managing Risks with Evolving Technologies
Host-cell proteins (HCPs) are major impurities of concern in biomanufacturing. When present in drug formulations, they can reduce efficacy (by compromising product stability), introduce toxicity, and increase a recipient’s risk for long-term immunogenicity. Understanding HCP profiles and integrating effective removal strategies are important parts of developing new biological drugs — to fulfill regulatory guidelines and to ensure patient safety through product quality. HCP populations can be both complex and structurally diverse, and some changes in upstream culture conditions can affect…
Stability Testing: Monitoring Biological Product Quality Over Time
Many physical and chemical factors can affect the quality, safety, and efficacy of biopharmaceutical products, particularly after long-term storage in a container–closure system that can be subject to variations in temperature and light, as well as agitation with shipping and handling. Proteins are inherently complex physiochemically, from their primary amino acid sequences to their higher-order structures, and they require specific conditions to maintain their integrity and functionality. Advanced biological therapies can be even more complicated and particular about their environments.…