Biosimilars have become common on pharmacy shelves in Europe. The first biosimilar product — Sandoz’s Omnitrope version of Lilly’s Humatrope (somatropin) — was approved by the European Medicines Agency (EMA) in 2006. In the decade that followed, more than 20 biosimilars have gained regulatory approval in Europe. The first biosimilar monoclonal antibodies (MAbs) — comparators to Janssen’s Remicade (infliximab) — were approved in 2013. The pace of approvals in the United States has been much slower. The US Food and…
Author Archives: Bruno Speder
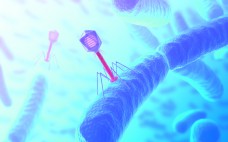
Bacteriophages, an Alternative to Antibiotics: Challenges and Possible Solutions for Bringing Them to Market
Bacteriophages are viruses (consisting of a genome contained within a protein coat) that specifically infect bacteria. They are the most abundant living entities on earth — the estimates range from 1030 to 1032 in total — and play key roles in regulating the microbial balance in every ecosystem where that has been explored (1). Bacteriophages are genotypic and phenotypically different from viruses that infect Archaea (Archaeovirus) and Eukarya (Eukaryovirus). The name bacteriovirus has been proposed as scientifically more accurate (2).…