Continued clinical efficacy demonstrations of cell-based immunotherapies (iTx) such as chimeric antigen receptor T cell (CAR-T) therapies has made the prospect increasingly likely of an immunotherapy product achieving conditional market authorization in the short term. For example, Novartis and the University of Pennsylvania’s lead candidate (CTL019) for treating a range of hematological malignancies received breakthrough status from the US Food and Drug Administration (FDA) in 2014, permitting access to an expedited drug development pathway for high unmet medical needs (1).…
Author Archives: Brock Reeve
Extracellular Vesicles Commercial Potential As Byproducts of Cell Manufacturing for Research and Therapeutic Use
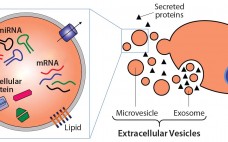
Extracellular vesicles (EVs) are emerging as a potential alternative to some stem-cell–derived therapeutics (1, 2). Sometimes called exosomes, they are small, secreted vesicles that can possess similar therapeutic mechanisms to whole cells, possibly representing the active pharmaceutical ingredient. In the past 15 years, academic and industry interest in EVs has exponentially increased as mounting evidence demonstrates their role in physiology and pathology as well as their therapeutic potential. In light of growing efforts in using EVs for research and therapy,…