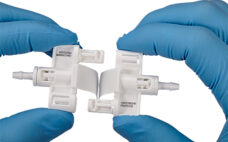
Small-volume biopharmaceutical processes continue to grow as more biopharmaceutical, cell therapy and gene therapy companies develop products. A 2021 Association for Regenerative Medicine (ARM) report states that there are 1,085 cell, gene and tissue-based therapy developers worldwide. These companies are engaged in many small-volume (<10L) processes including early-stage drug development and autologous therapies. The increase in small-volume processes coincides with the ongoing need to create fast, reliable aseptic closed systems. Historically, few convenient options have existed to facilitate sterile processing.…
Author Archives: BPI Staff
Pharmaron’s cell and gene therapy webinar series
Pharmaron has taken their platform beyond small molecules and are pleased to now offer a full array of services for biologics and cell and gene therapies (CGTs). Our new CGT webinar series focusses on identifying key cell and gene therapy questions that arise during drug discovery and development. Register here for the series. Our second webinar, entitled Downstream AAV Production: A Targeted Approach to Optimization takes place on Thursday, March 24 at 9.00am ET. Join us as we highlight our…
CMC Testing Support for Gene and Cell Therapy
With the promise to enhance treatment, greatly reduce side effects, and potentially cure many types of diseases and disorders, Advanced Therapy Medicinal Products (ATMP), such as gene and cell therapy products, are in high demand, and biopharma companies are in a race to the clinic. However, these technologies are very complex in nature and are vastly different than traditional biopharmaceutical products, especially when it comes to the use of these products for personalized medicine. The complexities span the development pipeline,…
Is the AAV payload impacting the stability of your gene therapy?
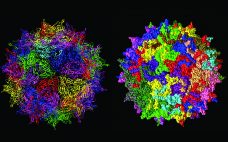
The ability to use adeno-associated viruses (AAVs) for targeted therapeutic payload delivery has dramatically accelerated the development of life-saving gene therapies. Development can further be accelerated by monitoring critical quality attribute parameters like subvisible particles (SVP) concentration early in the development process. Capsid degradation and nucleic acid leakage can both occur under different formulation and storage conditions and contribute to the accumulation of aggregates. Characterizing candidates earlier in the development process is best as it identifies and eliminates inherently unstable…
In brief: Vetter opens clinical filling site in Austria
Vetter has received manufacturing authorization for a fill and finish site Austria, which the CDMO acquired in 2020. Contract development and manufacturing organization (CDMO) Vetter bought the clinical production site in Rankweil, on the Austrian border with Switzerland and Liechenstein, from Impletio – part of Rentschler, with which Vetter inked an alliance in July 2020. The firm has been working to modify and equip all laboratory, technical and production areas at the 10,000 square-meter facility and has announced this week…
WACKER’s plug-and-play technology serves growing pDNA demand for cell and gene therapy
The global demand for nucleic acid-based gene therapies, novel vaccines and innovative therapeutic agents, including messenger RNA (mRNA) and viral vectors, is extremely high and projected to increase in the future. Plasmid DNA (pDNA) is the basis for all of these advanced therapies. Plasmid DNA can be used either directly for vaccines and gene therapies, or as starting material, e.g., to manufacture mRNA. The CDMO market for pDNA is expected to grow by 19 percent by 2025 (Global Viral Vector…
Univercells adds DNA synthesis through SynHelix buy
Univercells has entered the synthetic biology space through the acquisition of French DNA synthesis firm SynHelix. The deal, financials of which have not been disclosed, sees Belgian bioprocess firm Univercells add technology aimed at simplifying biotherapeutic development through a scalable and automated DNA synthesis platform. SynHelix’s technology is based on the GMP production of long DNA fragments in large quantities and has been touted as an alternative to DNA amplification on bacteria in the synthetic biology, gene therapy, and diagnostics…
BioProcess Insider’s Holiday Vaccine Hunt
It’s been another pandemic-dominated year but can you find all the COVID-19 vaccines hidden in the grid below? Bioprocess Insider’s Covid Word Search Puzzle » play word search
What’s new in AAV manufacturing? 3 takeaways from Thermo Fisher Scientific
From virtual regulatory inspections to optimization of scalable AAV production, the gene and cell therapy fields continued to innovate and evolve throughout the pandemic. Thermo Fisher Scientific hosted an interactive workshop highlighting some of these developments, including a new AAV production system, a custom media platform for viral production cell lines, and changes to the regulatory process. Read on to get a taste of those insights. A Helper-Free AAV Production System Adeno-associated viruses (AAVs) are among the most promising gene…
Increasing productivity in cell and gene therapy bioprocessing with fast and reliable immunoassays
Cell and gene therapy manufacturers need to ensure the safe delivery of their products and rely on fast and reliable immunoassay platforms to do so. This white paper gives an overview of the viral vector landscape and the requirements for immunoassays in viral vector bioprocessing analytical workflow. It also shares case studies from viral vector labs that are using the Gyrolab technology to deliver fast and reliable results. Increasing productivity in cell and gene therapy bioprocessing with fast and reliable…