Compared with that for new drugs, biosimilar development faces significantly condensed timelines from cell line to first-in-human (FIH) trials. A biosimilar development program needs to accelerate quickly toward preclinical and phase 1 studies; phase 2 studies typically are not required because dose response and other patient-treatment concepts are already established by the original, comparator medicine. Phase 3 studies typically are limited to fewer patients, which ultimately shortens overall timelines and costs. The key challenge remains: demonstrating comparability and high similarity…
Author Archives: David Calabrese
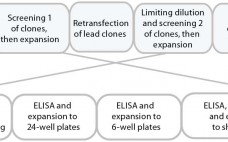
Rapid Development and Scale-Up Through Strategic Partnership: Case Study of an Integrated Approach to Cell-Line and Process Development for Therapeutic Antibodies
Over the past decade, monoclonal antibodies have become mainstream therapeutics for treating a broad range of conditions from autoimmune disorders to cancer. Part of this evolution is increasing time and cost pressure on biopharmaceutical companies to bring new drugs to market 1, 2. Additionally, companies now routinely engineer and screen molecules for developability and manufacturability during discovery before selecting a final candidate molecule. The biosimilar development paradigm also demands significantly more bioanalytical analysis during initial cell-line and process development. Thus,…
Rapid Production of Functional Proteins of a Combinatorial IgG Library in CHO Cells
Recombinant DNA (rDNA) technologies provide a wide range of tools for producing a broad array of recombinant proteins. Since the early 1970s, the biotechnology industry has harnessed those tools — together with genetic engineering and genomics — for developing new classes of innovative and effective therapeutic molecules. The therapeutic recombinant protein market segment now represents the core of the medical biotechnology industry, with hundreds of companies involved in discovery, development, and marketing. Although recombinant technologies are extremely powerful tools, significant…